Chapter 23
Examination of the Urinary Sediment
Specimen Collection
In addition to the biologic variability of patients, urinalysis results are influenced by the urine collection method, the timing of urine collection, administration of therapeutic or diagnostic agents prior to collection, and how the sample is handled prior to analysis.1 Ideally, at least 6 milliliters (mL) of urine should be collected prior to the administration of therapeutic or diagnostic agents to establish baseline information; however, in patients with cystitis and urge incontinence, this ideal may be challenging to attain. In urinalysis, 5 mL of urine may be used; 1 mL may be used for urine culture, if necessary. When choosing the urine collection method and the timing of urine collection, it is useful to consider the patient’s clinical status, the logistics of the collection method, and the intended use of the sample (Table 23-1 and Table 23-2).
TABLE 23-1
Methods of Urine Collection with Their Advantages and Disadvantages or Precautions
| Collection Method | Advantages | Disadvantages / Precautions |
| Midstream, naturally voided | Noninvasive, relatively easy technique in dogs May be performed by clients and is useful to collect first morning, maximally concentrated urine samples from outpatients Unlike cystocentesis or catheterization, is not associated with iatrogenic hematuria Though not ideal, a freshly voided sample may be used for urine culture, as long as a quantitative urine culture is performed | Likely contaminated by a variable amount of material from the lower genitourinary tract (e.g., bacteria, epithelial cells, blood, sperm, debris), perineum, or environment (e.g., pollen), which may be observed in the urine sediment. Cleanser residues or microorganisms within the collection vessel may affect results. Urine should be collected into a sterile, single-use urine collection cup, rather than into a reusable container. Avoid manual bladder compression to induce micturition, which may cause reflux of urine into other organs (e.g., kidneys, prostate) or iatrogenic hematuria. |
| Transurethral catheterization | Useful collection method when an indwelling urinary catheter is already present for another reason Though not ideal, sample may be used for urine culture, as long as a quantitative urine culture is performed | Risk of traumatic catheterization, which may injure the patient and contaminate the sample with blood. Risk of iatrogenic infection, especially in patients predisposed to urinary tract infection (e.g., lower urinary tract disease, renal failure, diabetes mellitus, hyperadrenocorticism). Should be performed aseptically and atraumatically by a trained, experienced individual. Urine sample may be contaminated by variable numbers of epithelial cells, bacteria, and debris from the lower genitourinary tract, which may be observed in the urine sediment. Catheters that are chemically sterilized may contain residue of the antiseptic solution, which may irritate mucosal linings and affect results of urinalysis and urine culture. Catheterization may be technically challenging in female patients. May require use of a vaginal endoscope. |
| Antepubic cystocentesis | Avoids lower genitourinary tract contamination of urine sample Ideal sample for urine culture Less risk of iatrogenic infection compared with transurethral catheterization Easier than collection of a voided sample from cats Better tolerated than catheterization | Contraindicated in patients with urethral obstruction or bleeding diathesis (e.g., thrombocytopenia), may be performed with caution after cystotomy. An adequate volume of urine within the bladder is required. Blind cystocentesis without at least manual localization and immobilization of the bladder is not recommended. Ultrasound-guided needle placement is helpful, though not mandatory. Misdirection of the needle may lead to a nondiagnostic or contaminated sample (e.g., enterocentesis). A variable degree of iatrogenic microscopic hematuria, which cannot be readily distinguished from pathologic, disease-induced hematuria, may be caused by this collection method. This type of contamination may be particularly pronounced when the bladder wall is inflamed or congested. Iatrogenic hematuria may limit the utility of this collection method when monitoring the progression of disease in a patient that has pathologic hematuria. |
TABLE 23-2
Timing of Urine Sample Collection, Indications, and Potential Effects on Urinalysis Results
| Collection Time | Advantages | Disadvantages |
| First morning urine – Urine is formed after a several hour period of nil per os | Represents the patient’s maximally concentrated urine and is, therefore, ideal for assessing renal tubular ability to concentrate urine Microscopic sediment will be more concentrated Postprandial alterations unlikely Urine more likely to be acidic, so casts may be better preserved (proteinaceous structures dissolve in alkaline urine) | Urine present within bladder for a relatively prolonged period May alter cellular morphology observed during microscopic examination May decrease viability of fastidious microorganisms, causing false-negative culture results |
| Postprandially | Useful to assess the effect of diets intended to modulate urinary pH when collected 3 to 6 hours postprandially More likely to detect hyperglycemic glucosuria when collected 3 to 4 hours postprandially | pH may be elevated by postprandial alkaline tide when collected within 1 hour postprandially |
| Randomly timed urine sample – Represents urine that has accumulated within the bladder for minutes to hours or urine that has been diluted by recent ingestion of water | Cytomorphology and viability of fastidious microorganisms may be better preserved since urine is stored within the bladder for relatively less time | If the urine is isosthenuric or minimally concentrated, no conclusion can be drawn about renal tubular concentrating ability |
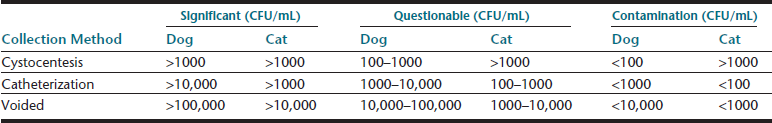
Samples collected during natural, midstream micturition or by transurethral catheterization are also suitable for sediment examination and a specialized type of culture—quantitative urine culture—which should be interpreted using guidelines based on collection method and colony-forming units per milliliter (CFU/mL) (Table 23-3). Manual compression of the bladder to induce micturition should be avoided, since doing so may cause reflux of potentially infectious urine, traumatic hematuria, or rarely uroabdomen. Voided urine samples rescued from the examination room tabletop have limited utility; but, if the sediment is examined without delay, some components may still be assessed, specifically cells that might come from the patient (e.g., leukocytes, erythrocytes, atypical cells). Such a sample should not be used for biochemical analysis or to screen for bacteriuria.
In addition to routine urine sediment evaluation, urine samples may be converted to a dry-mount cytology;2,3 this permits more sensitive detection of bacteria and more accurate assessment of bacterial morphology and greatly facilitates evaluation of atypical cells in-house or by a reference laboratory. The method is described in Box 23-1. If available, cytocentrifugation of urine sediment is equally useful. When applicable, obtaining cells directly from a mass (i.e., traumatic catheterization, ultrasound-assisted fine-needle biopsy [FNB], or surgical biopsy imprint) usually produces a sample with the best morphology for cytologic examination.
Specimen Handling Prior to Urinalysis
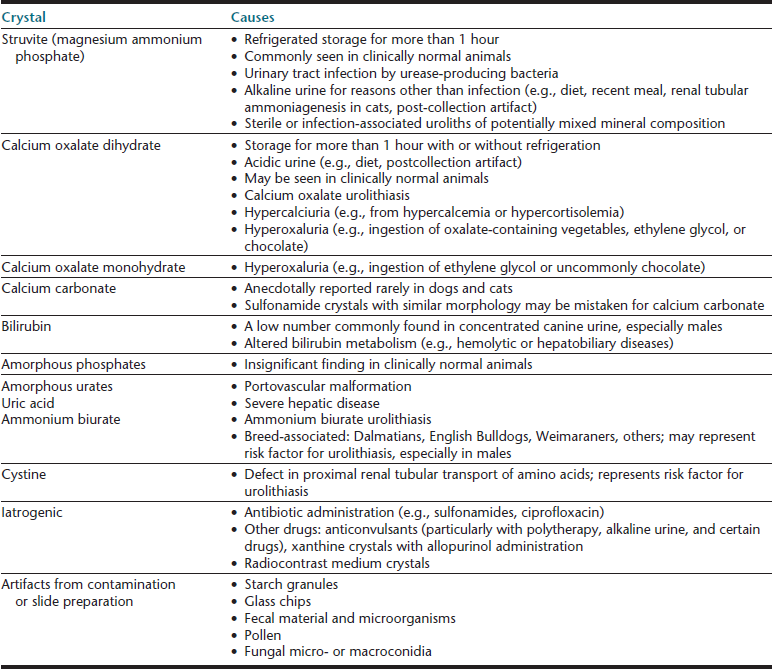
With proper sample handling and testing, complete urinalysis may rapidly provide information about the genitourinary tract and screen for diseases of other body systems (e.g., endocrine, hepatic). Urine should be collected into a sterile, single-use vessel to avoid potential contamination by cleanser residues and microorganisms. The body of the container (not just the lid) should be labeled, and the container should be sealed to avoid sample leakage and evaporation of volatile compounds (e.g., ketones). To minimize postcollection artifacts and obtain results that are most representative of urine in vivo, urine samples should be evaluated within 30 minutes of collection.4 If urinalysis will be delayed, the sample should be refrigerated and protected from light to prevent overgrowth of microorganisms and photodegradation of bilirubin, respectively. If necessary, samples may be stored for approximately 12 to 24 hours (i.e., overnight); however, depending on the initial sample composition (e.g., pH, concentration of crystallogenic substances), the sediment content may be modified from what was initially present immediately ex vivo—crystals may form with refrigerated storage (i.e., struvite, calcium oxalate dihydrate), renal tubular casts may degrade, cytomorphology may be detrimentally altered.5 Freezing or routine use of chemical preservatives should be avoided. Refrigeration is the preferred means to preserve urine samples; however, since cold urine may influence urinalysis results (e.g., falsely increase specific gravity, inhibit enzymatic urine dipstick reactions, promote crystal formation), a sample that has been refrigerated should be permitted to warm to room temperature prior to urinalysis. If crystalluria is a medically important problem that is being evaluated, then the finding should be confirmed in a freshly obtained sample collected into a single-use container and analyzed within 30 to 60 minutes without intervening refrigeration.4,5
Preparation of Urine Sediment Wet-Mount
The urine sample should be mixed well prior to removing an aliquot for centrifugation to avoid loss of formed elements by spontaneous sedimentation that may have occurred prior to urinalysis. Most urine sediment findings are reported semiquantitatively, though the coefficient of variation is high for microsopy.6 To aid result interpretation and intersample result comparisons, laboratories should use a standardized technique for urine sediment preparation and evaluation (Box 23-2). The starting volume of urine used to prepare the sediment, the speed and duration of centrifugation, and the volume in which the sediment pellet is resuspended after centrifugation affect the concentration of the urine sediment. Ideally, both a standard starting volume of urine and a standard volume to resuspend the sediment pellet should be used. When possible, 5 mL of well-mixed urine should be centrifuged at 400 to 500 times gravity (g) (approximately 1000–1500 revolutions per minute [rpm]) in a conical centrifuge tube for 5 minutes; 4.5 mL of supernatant should be removed and either discarded or saved for subsequent biochemical testing (e.g., urine specific gravity, sulfosalicylic acid precipitation of protein). The sediment pellet should be gently resuspended in 0.5 mL of supernatant, which represents 10% of the original starting volume. When restricted by lesser urine sample volume, the volume of supernatant used to resuspend the pellet for wet-mounting should accordingly be reduced to 10% of the initial volume of urine centrifuged (see Box 23-2). Commercial systems for urine sediment preparation (e.g., Stat-Spin®, Kova®, IRIS International, Inc., Chatsworth, CA; HYCOR Biomedical, Indianapolis, IN, respectively) are available. Adherence to the manufacturer’s guidelines will ensure standardized results. The urine sediment pellet may be gently resuspended by using a disposable transfer pipet to gently aspirate and expel the contents of the centrifuge tube to form a suspension or by holding the conical centrifuge tube between an index finger and thumb to form a fulcrum and gently flicking the tube with the contralateral index finger to create a weak vortex. Aggressive mixing may degrade fragile structures such as casts.
To prepare an unstained wet-mount, a single drop of the well-mixed pellet suspension should be transferred to a clean glass microscope slide and a coverslip applied. A stained wet-mount prepared using a drop of 0.5% new methylene blue or Sternheimer-Malbin stain (Sedi-Stain™, Becton Dickinson, Rutherford, NJ) added to the pellet suspension is helpful in identifying nucleated cells. Addition of stain will dilute the concentration of the sediment or may contaminate the sample with crystals or microorganisms. Semiquantitative results should be determined using an unstained wet-mount, and any crystals or microorganisms that are identified in a stained wet-mount should be confirmed in an unstained wet-mount. It may be useful to mount a stained wet-mount and an unstained wet-mount side by side on a single slide. In general, wet-mounts should be examined without delay. However, a humidified chamber prepared from a Petri dish, a thin layer of dampened absorbent material, and a halved cotton-tip applicator stick (Figure 23-1) may be used to temporarily preserve a wet-mount in instances when consultation with an in-house colleague is desired.
Microscopic Examination of Urine Sediment
To enhance visualization of materials in wet-mounts, samples are observed with modified illumination to increase the refraction of formed elements relative to the surrounding liquid. Proper illumination using a light microscope is achieved by either lowering the substage condenser a couple of centimeters or by partial closure of the iris diaphragm of the condenser; the latter is considered optically superior. In addition to scanning the entire coverslip, a standardized number of 10× and 40× microscopic fields, that is, 10 fields at each magnification, should be examined to determine the concentration of formed elements. Box 23-3 details steps for systematic microscopic urine sediment evaluation and reporting.
Urinary Sediment Findings
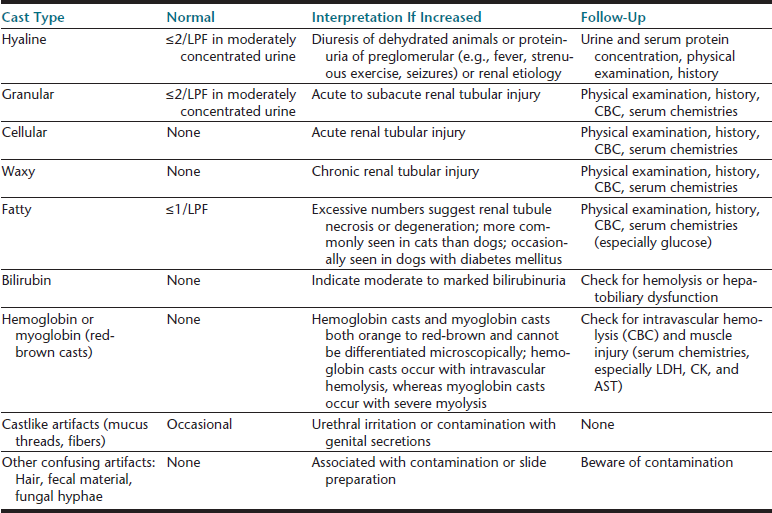
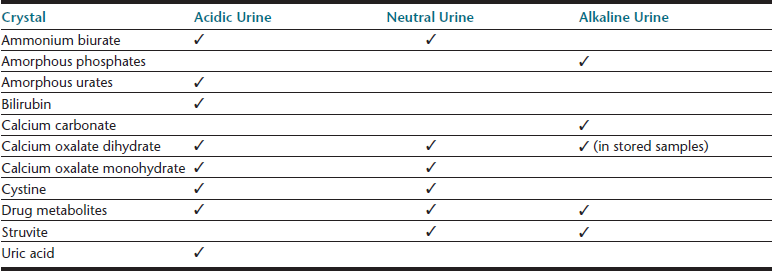
Cells (Table 23-4), microorganisms (see Table 23-4), casts (Table 23-5), crystals (Table 23-6 and Table 23-7), lipid, and contaminating substances may be found in urinary sediment. Urine obtained from healthy dogs and cats forms little sediment. Small numbers of epithelial cells, mucous threads, erythrocytes, leukocytes, hyaline casts, and various types of crystals may be found in the urine of most healthy animals. Bacteria and squamous epithelial cells derived from external genital surfaces may be present in voided and catheterized urine. When interpreting urine sediment, it is also necessary to keep in mind the biochemical findings that may influence the sediment content, for example, in dilute urine (specific gravity <1.008) erythrocytes will likely be lysed; highly alkaline urine may reduce the numbers of cells and casts; and urine pH influences crystal formation.
TABLE 23-4
Routine Microscopic Urine Examination: Cells, Cell-like Structures, Microorganisms, Parasites, and Confusing Artifacts
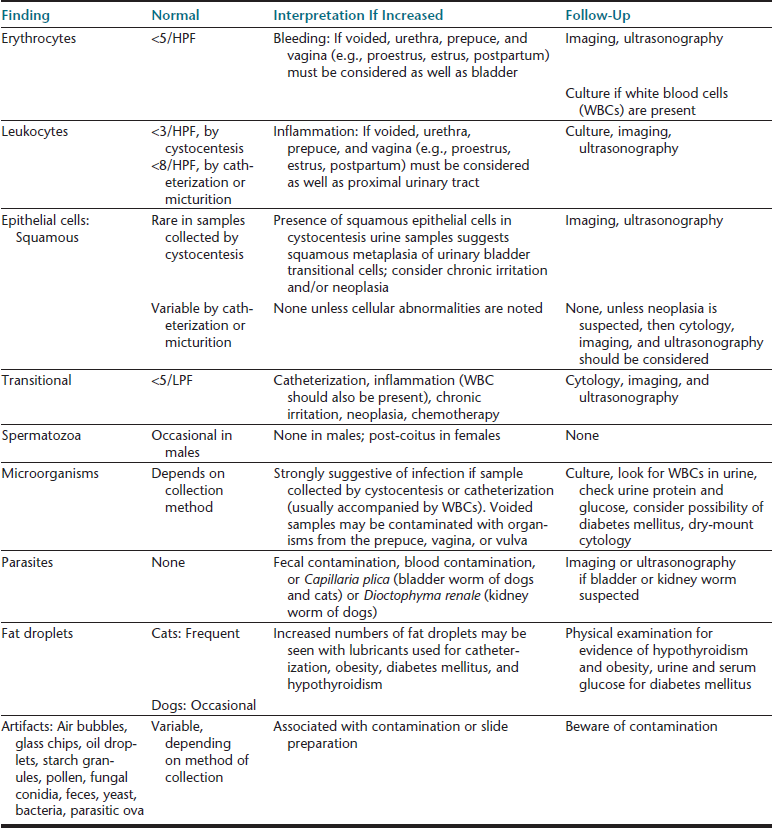
HPF, high-power field (40× objective); LPF, low-power field (10× objective).
Epithelial Cells
Squamous Epithelial Cells
Squamous epithelial cells line the distal third of the urethra, the vagina, and the prepuce and are usually not considered significant. They are the largest of the epithelial cells and the largest cell from the patient found in urine sediment. They are flat or rolled cells, which have at least one angular border and usually a single small, condensed nucleus, or they may be anucleate (Figure 23-2, Figure 23-3; Figure 23-16; Figure 23-17; Figure 23-18).
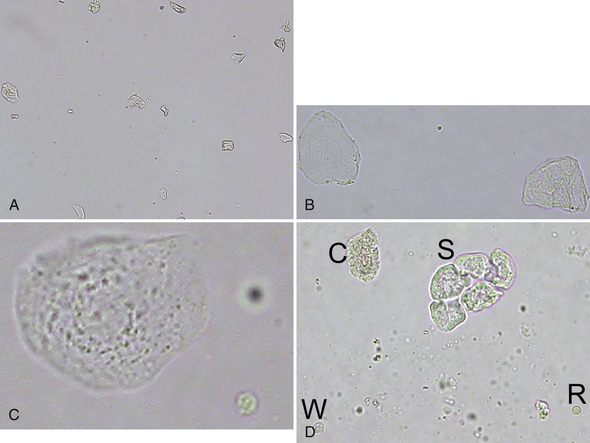
Figure 23-2 A, Voided urine sediment with squamous epithelial cells, which are lying flat or partially twisted. (Unstained. Original magnification 100×.) B, Two anucleate squamous epithelial cells with one of more angular borders. A small lipid droplet is in the center. (Unstained. Original magnification 400×.) C, Nucleated squamous epithelial cell with granular cytoplasm with a crenated erythrocyte (lower right). Note the relative size of these cells. (Unstained. Original magnification 400×.) D, A sheet of squamous epithelial cells (S), a leukocyte (W), an erythrocyte (R), and a granular cast fragment (C) (same dog as in Figure 23-40). The erythrocyte is biconcave and more translucent than the epithelial cells and the leukocyte. Small, refractile amorphous crystals are scattered in the background. (Unstained. Original magnification 400×.)
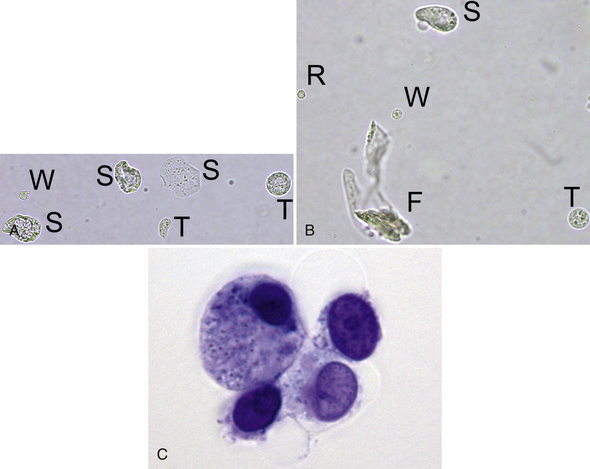
Figure 23-3 A, Two transitional epithelial cells (T), three squamous epithelial cells (S), and one leukocyte (W). Note the relative size and varied appearance of these cells. (Unstained. Original magnification 400×.) B, Note the relative size and appearance of a rounded squamous epithelial cell (S), a transitional epithelial cell (T), a leukocyte (W), and an erythrocyte (R). A segmented nucleus is visible in the leukocyte, and the erythrocyte is a ghost. A contaminating fiber (F) is present in several focal planes. (Unstained. Original magnification 400×.) C, Four variably sized transitional epithelial cells. (New methylene blue stain. Original magnification 1000×.)
A variable number of squamous epithelial cells are most commonly observed with lower genitourinary tract contamination of voided or catheterized samples. Squamous epithelial cells are typically not present in samples collected by cystocentesis. A significant number of squamous epithelial cells are very rarely seen in cystocentesis samples because of squamous cell carcinoma of the bladder or squamous metaplasia of the bladder, which may occur with transitional cell carcinoma or chronic bladder irritation. With transitional cell carcinoma, many other features suggestive of epithelial neoplasia, including an extreme number of epithelial cells with marked pleomorphism, are typically found. Squamous epithelial cells may also be found if the uterine body of an intact female is unintentionally penetrated during cystocentesis. Cells with squamous features may be found in the urine sediment of male dogs with conditions that cause squamous metaplasia of the prostate.
Transitional Epithelial Cells
Transitional epithelial cells line the proximal two thirds of the urethra, bladder, ureters, and renal pelves. They are highly pleomorphic, variably sized cells that are smaller than squamous epithelial cells and two to four times larger than leukocytes; those originating in the proximal urethra and bladder are the largest. They may be round, oval, pear-shaped, polygonal, or caudate and often have granular cytoplasm with a single nucleus that is larger than that of squamous epithelial cells (see Figure 23-3; Figure 23-22; Figure 23-39; Figure 23-51.
There should be less than 5 transitional epithelial cells per 10× low-power field in normal urine sediments. A greater number of transitional epithelial cells are seen in urine samples collected by catheterization or in patients with inflamed, hyperplastic, or neoplastic mucosa. Urine from animals with inflammation-induced epithelial hyperplasia also contains increased numbers of leukocytes (Figure 23-4). Urolithiasis and some chemotherapeutic agents (e.g., cyclophosphamide) may induce epithelial hyperplasia with mild to moderate atypia (Figure 23-5). Many epithelial cells are found in sediment from animals with transitional cell carcinomas, and a variable number of leukocytes are also often present because of secondary inflammation or infection. Neoplastic epithelial cells are found individually or in large cohesive sheets, tend to be larger than normal epithelial cells, usually vary markedly in size, and have a high nuclear to cytoplasm ratio along with other malignant features (Figure 23-6). Knowledge of the clinical context is important when determining the significance of atypical epithelial cells in urine sediment, for example, presence of uroliths, presence and location of urinary tract mass, or medication administration.
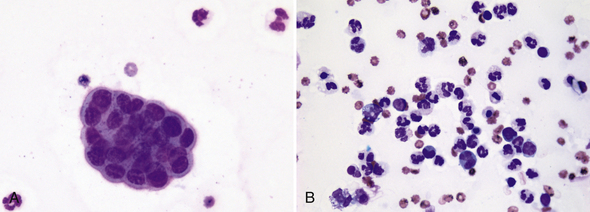
Figure 23-4 Urine sediment dry-mount cytology from a Labrador Retriever with chronic cystitis.
A, A tightly cohesive, mildly atypical, small sheet of transitional epithelial cells with high nucleus-to-cytoplasm ratio, but minimal size and shape variation is present with three mildly degenerate neutrophils. B, Another microscopic field from the same sample that demonstrates mixed inflammation. A few of the neutrophils are pyknotic. (Wright-Giemsa stain. Original magnification both images 500×.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree