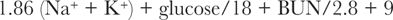Chapter 45 Ethylene Glycol
Ethylene glycol (EG) poisoning is common in dogs and cats1–4 and often results in death if it is not diagnosed and treated promptly.5 The mortality rate in dogs is reported to range from 59% to 70%1,5 and is thought to be even higher in cats. EG intoxication is the second most common cause of fatal poisoning in animals according to the American Association of Poison Control Centers.6 This high incidence is due to the ready availability, pleasant taste, and small quantity of EG necessary to induce poisoning. Bittering agents have been added to some EG formulations to deter ingestion. It is not known how effective this has been in reducing the number of poisonings in pets. Although most EG poisonings are accidental, malicious poisonings also occur. The incidence is also relatively high in humans, with approximately 5000 episodes reported in the United States each year.7,8 The vast majority of these are unintentional, and approximately one third of the cases occur in children.7 EG is the most common cause of human poisoning in some countries, such as Poland.9 The first reported case of EG intoxication in a human being occurred in 1930,10 but the toxicity of EG was not fully realized until 1938 when 76 persons died after consuming an elixir of sulfanilamide containing 96% diethylene glycol.11 Since then many reports of EG poisoning in humans and other animals have been published.3,12–30
SOURCES
EG (C2H6O2) is a colorless, odorless, sweet-tasting liquid used primarily as an antifreeze and windshield de-icing agent. Its small molecular weight (62 Da) makes it effective in lowering the freezing point of water. EG is also used as a cryoprotectant for embryo preservation, in the manufacture of polyester compounds, as a solvent in the paint and plastic industries, and as an ingredient in photographic developing solutions, hydraulic brake fluid, motor oil, inks, paints, and wood stains.31 The most readily available source of EG in the home is antifreeze solution, which consists of approximately 95% EG. All dogs and cats that have been brought to Colorado State University with EG toxicosis are thought to have ingested antifreeze, with the exception of one cat that ingested photographic developing solution.3 The source of antifreeze is usually an open container or a puddle of antifreeze that has drained from a radiator. Rarely, dogs may chew open a plastic container of antifreeze. Intoxication occurs most commonly in the fall, winter, and spring, the seasons in which antifreeze is most commonly used.3,26
TOXIC DOSE
The minimum lethal dose of undiluted EG is 6.6 mL/kg in the dog32 and 1.5 mL/kg in the cat.33
TOXICOKINETICS
Following ingestion, EG is rapidly absorbed from the gastrointestinal tract; the rate of absorption is delayed when food is in the stomach.34 It is then quickly distributed throughout the blood and tissue. The plasma half-life of EG is approximately 3 hours.35,36 A variable amount, depending on dose and species, is eliminated unmetabolized in the urine.35,37,38 The remaining EG is metabolized, primarily by the enzyme alcohol dehydrogenase (ADH) and other hepatic enzymes, to glycoaldehydes and organic acids. The elimination system appears to be saturable, with semilogarithmic rates of elimination at low doses giving way to zero-order elimination kinetics at higher doses. Metabolites are present for up to several days, and calcium oxalate is present in tissue for much longer.31
The liver is the major site of EG metabolism, although small amounts of ADH are present in other organs, such as the kidney and stomach.39,40 EG is initially oxidized to glycoaldehyde by ADH, and glycoaldehyde is then oxidized to glycolic acid and then to glyoxylic acid. Glyoxylic acid is primarily converted to oxalic acid, but may follow several metabolic pathways; end products may also include glycine, formic acid, hippuric acid, oxalomalic acid, and benzoic acid. EG and glycolic acid are excreted in urine in higher quantities than any other metabolites because their me-tabolism is rate limiting.41,42 Calcium is bound to oxalic acid, resulting in calcium oxalate crystal formation. Calcium oxalate crystal deposition is widespread, but is most severe in the kidney, and crystalluria is a consistent finding in animals producing urine.34,43
MECHANISM OF TOXICITY
Before it is metabolized, EG is no more toxic than ethanol, although EG is a more potent central nervous system (CNS) depressant than is ethanol.44 EG per se has no major effects other than gastrointestinal irritation, increased serum osmolality, and CNS depression. However, unlike ethanol, EG is biotransformed to highly toxic metabolites that result in severe metabolic acidosis and acute renal failure, which are hallmarks of EG poisoning.3,31,45
Cytotoxicity
The cytochrome P450 system is partially responsible for and is inhibited by the metabolism of EG. Electron transfer within the P450 system is affected, resulting in the production of oxygen radicals that are probably toxic to tissue. Moreover, the organic acid metabolites inhibit oxidative phosphorylation, cellular respiration, glucose metabolism, protein synthesis, and DNA replication.46,47 The wide range of tissue toxicities seen in animals with EG toxicosis may be due to the fact that different tissues use different isoenzymes of the cytochrome P450 family.
Gastrointestinal system and liver
EG is a gastric irritant that commonly results in vomiting in dogs and cats.26 Nausea, vomiting, hematemesis, abdominal pain, and cramping have been associated with EG ingestion in human beings.31 Calcium oxalate deposits and focal hemorrhages may be found in the gastric mucosa at necropsy. Ultrastructural evidence of hepatocellular damage has been reported,48,49 although serum biochemical and histopathological evidence of hepatotoxicity is not usually associated with EG poisoning in dogs and cats.
Nervous system
Glycoaldehyde is thought to be the metabolite primarily responsible for CNS dysfunction; respiration, glucose, and serotonin metabolism are depressed, and CNS amine concentrations are altered.19,22 Marked cerebral edema is commonly seen during the later stages of EG poisoning in human beings.50 Calcium oxalate deposition, hemorrhages, perivascular infiltration, and neuronal degeneration may be present.51 Hypocalcemia secondary to calcium oxalate deposition may contribute to CNS signs, although the concurrent metabolic acidosis shifts calcium to the ionized active state, reducing the chances of hypocalcemia-associated clinical signs. Acidosis is also thought to lead to altered levels of consciousness and cerebral damage.
Metabolic acidosis
Metabolic acidosis is often severe and has a deleterious effect on multiple organ systems. Glycolic acid accumulation is the primary cause of the metabolic acidosis associated with EG intoxication,52 although other acid metabolites also contribute. Glycolic acid accumulates because the lactic dehydrogenase enzyme that metabolizes glycolic acid to glyoxylic acid becomes saturated.
Renal failure
Renal failure is the most profound consequence of EG poisoning in dogs and cats. In human beings, permanent renal failure, as evidenced by tubular atrophy and interstitial fibrosis, is rare; renal function is usually restored within 2 months after EG ingestion. However, dogs and cats are rarely maintained for this period of time and are commonly euthanized during the anuric or oliguric stage of acute renal failure.26 Most of the metabolites are cytotoxic to renal tubular epithelium, and some renal epithelial and interstitial damage may be associated with calcium oxalate crystal formation within the renal tubules.53 Renal epithelial cell death appears to be caused primarily by destruction of cytoplasmic organelles, especially mitochondria.46
CLINICAL SIGNS
Clinical signs are dose dependent and can be divided into those caused by unmetabolized EG and those caused by the toxic metabolites of EG. The onset of clinical signs is almost always acute. Early clinical signs are usually observed 30 minutes after ingestion and often last until approximately 12 hours after ingestion; they are primarily associated with EG-induced gastric irritation and high EG blood concentrations. These signs commonly include nausea and vomiting, CNS depression, ataxia and knuckling, muscle fasciculations, decreased withdrawal reflexes and righting ability, hypothermia, and osmotic diuresis with resultant polyuria and polydipsia.3,26,34 As CNS depression increases in severity, dogs drink less, but osmotic diuresis persists, resulting in dehydration. In dogs CNS signs abate after approximately 12 hours, and patients may briefly appear to have recovered. Cats usually remain markedly depressed and do not exhibit polydipsia. Animals may be severely hypothermic, particularly if housed outside during the winter months.
MINIMUM DATABASE
Complete blood count (CBC)
The CBC is not particularly useful in the diagnosis of EG poisoning. Hematological abnormalities, when present, are associated with dehydration (increased packed-cell volume and increased plasma protein concentration) and stress (mature neutrophilia and lymphopenia).3,26 Erythrocytes occasionally exhibit echinocytosis. The mechanism for this is not understood and may be related to abnormal serum electrolyte concentrations or increased serum osmolality.
Serum biochemical profile abnormalities associated with early ethylene glycol intoxication
Abnormalities are primarily due to the presence of acid metabolites of EG in the serum that result in metabolic acidosis and include decreased plasma bicarbonate concentration and an increased anion gap. Additionally, hyperphosphatemia may occur because of ingestion of a phosphate rust inhibitor present in some commercial antifreeze products.26,34 The decreased plasma bicarbonate (HCO3−) concentration (sometimes reported as total CO2 on biochemical profiles) can be seen as early as 1 hour following EG ingestion.
Metabolites of EG notably increase the pool of unmeasured anions and cause an increased anion gap. The anion gap (the mathematical difference between measured anions and measured cations) is usually reported on biochemical profiles, but if not it can be calculated by subtracting the sum of the HCO3− (or total CO2) and chloride (Cl−) concentrations from the sum of the sodium (Na+) and potassium (K+) concentrations. For example, if a patient has an Na+ value of 155 mEq/L, K+ of 6 mEq/L, Cl− of 110 mEq/L, and HCO3− of 10 mEq/L, the anion gap is 41 mEq/L. Under normal conditions, the anion gap for dogs and cats is 8 to 25 mEq/L and 10 to 27 mEq/L, respectively, and is composed of phosphates, sulfates, and negatively charged proteins that are not included in the equation. The anion gap is increased by 3 hours after ingestion, peaks at 6 hours after ingestion, and remains increased for approximately 48 hours.43
Biochemical profile abnormalities associated with late ethylene glycol poisoning
With the onset of renal damage and subsequent decreased glomerular filtration, serum creatinine and blood urea nitrogen (BUN) concentrations increase. In the dog, these increases begin to occur between 24 and 48 hours following EG ingestion. In the cat, BUN and creatinine begin to increase approximately 12 hours after ingestion. Since polydipsia does not develop in cats, this may be in part due to dehydration. Serum phosphorus concentrations increase because of decreased glomerular filtration, but increases as high as 10 mg/dL may also be observed 3 to 6 hours following EG ingestion because of the phosphate rust inhibitors present in antifreeze solutions. In these cases, serum phosphorus concentrations return to normal and then increase again with the onset of azotemia. It is important to realize that hyperphosphatemia in the absence of an increased BUN or creatinine is most likely due to increased intake and is not an indication of compromised renal function. Hyperkalemia develops with the onset of oliguria and anuria.
A decrease in serum calcium concentration is observed in approximately half of patients3,26 and is due to chelation of calcium by oxalic acid. Clinical signs of hypocalcemia are infrequently observed because acidosis results in a shift to the ionized, physiologically active form of calcium.
Increased serum glucose concentration is also observed in approximately 50% of dogs and cats3,26 and is attributed to the inhibition of glucose metabolism by aldehydes, increased epinephrine and endogenous corticosteroids, and uremia.
Urinalysis
Dogs are isosthenuric (urine specific gravity of 1.008 to 1.012) by 3 hours following ingestion of EG because of osmotic diuresis and serum hyperosmolality-induced polydipsia.34,43 The urine specific gravity in cats is also decreased by 3 hours after ingestion, but may be above the isosthenuric range.54 Animals remain isosthenuric in the later stages of toxicosis because of renal dysfunction and an impaired ability to concentrate urine.
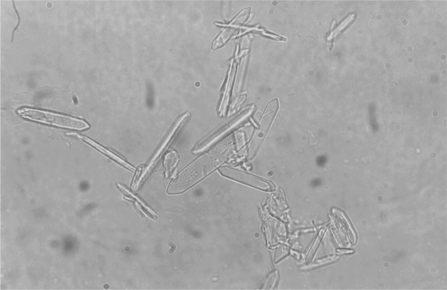
Calcium oxalate crystalluria is a common finding and may be observed as early as 3 to 6 hours after ingestion in the cat and dog, respectively, as a result of oxalic acid combining with calcium.43,54 Calcium oxalate monohydrate crystals are variably sized, clear, six-sided prisms (Figure 45-1). In animals and people poisoned with EG, the monohydrate form is observed more frequently than the dihydrate form, which appears as an envelope or Maltese cross.26,29 Dumbbell or sheaf-shaped crystals are observed infrequently. The monohydrate form was previously considered unusual in EG poisoning and was likely to be misidentified as hippuric acid crystals.3,55–59 Not only do monohydrate calcium oxalate crystals resemble hippuric acid crystals, theoretical arguments have supported hippuric acid crystal formation in patients with EG toxicosis.58,59 X-ray diffraction, however, has definitively identified the needle-shaped crystals as the monohydrate form of calcium oxalate rather than hippuric acid.60–63 The detection of calcium oxalate crystalluria, particularly the monohydrate form, provides strong supporting evidence for the diagnosis of EG poisoning.64 Thus urine microscopy is an important adjunct in the diagnosis of EG poisoning.
Urine pH consistently decreases following EG ingestion. Inconsistent findings include hematuria, proteinuria, and glucosuria. Granular and cellular casts, white blood cells, red blood cells, and renal epithelial cells may be observed in the sediment of some patients.3,26
CONFIRMATORY TESTS
Serum ethylene glycol concentration
EG serum concentrations peak 1 to 6 hours following ingestion, and EG is usually no longer detectable in the serum or urine 48 to 72 hours after ingestion.34,43,64a Inexpensive commercial kits* are available that accurately estimate blood EG concentrations at greater than or equal to 50 mg/dL, and the results correlate well with other established methods of measuring EG concentrations, such as gas chromatography,65 although the presence of propylene glycol or glycerol in the blood may cause a false-positive test result (some activated charcoal suspensions, formulations of diazepam, and semimoist diets contain propylene glycol). Ethanol and methanol do not result in a false-positive test result. Some hospitals and diagnostic laboratories can determine quantitative concentrations quickly enough to be diagnostically useful using enzymatic assays, although markedly increased concentrations of serum lactate dehydrogenase and lactic acid may result in a false-positive EG concentration result.29 Laboratory measurement of serum glycolic acid concentration, although diagnostically useful, particularly after EG has been metabolized, is rarely available at reference laboratories.66 Cats may be intoxicated with a lethal dose of EG that is still below the 50 mg/dL detectable level of the EG test kit. Therefore if the test kit result is negative and historical findings and clinical signs are compatible with EG ingestion, the recommendation is to initiate appropriate therapy for EG intoxication and submit a serum sample to a reference laboratory capable of determining a quantitative concentration.
Serum osmolality
Determination of serum osmolality is very useful for diagnosing early EG toxicosis.67 Serum osmolality is increased by 1 hour after ingestion of EG, increasing in parallel with serum EG concentrations.43 Hyperosmolality occurs because EG is an osmotically active, small molecular weight substance. When measured serum osmolality (by osmometry) is compared with calculated serum osmolality, the difference is referred to as the osmole gap. If calculated osmolality is not provided on the biochemical profile printout, osmolality in mosm/kg may be calculated using the following formula:
Normal serum osmolality is 280 to 310 mosm/kg, and the normal osmole gap is less than 10 mosm/kg. Serum osmolality as high as 450 mosm/kg serum and an osmole gap as high as 150 mosm/kg serum may be seen 3 hours after ingestion, depending on the quantity of antifreeze ingested.34,68 Both the gap and the measured osmolality may remain notably high for approximately 18 hours after ingestion. Multiplication of the osmole gap by five yields an approximate serum EG concentration in milligrams per deciliter.69 More specifically, each 100 mg/dL (16 mmol/L) increment increase in EG concentration contributes approximately 16 mosm/kg of H2O to the serum osmolality.29 Simultaneous or sequential increases in osmole and anion gaps are very suggestive of EG intoxication. As EG is metabolized, its contribution to the osmole gap diminishes because the accumulating negatively charged metabolites do not contribute to the osmole gap.29 Animals presenting with signs of late EG poisoning are likely to have little to no osmole gap increase, but will have an increased osmolality (whether calculated or measured) because of the azotemia.
Two types of instruments are used to measure osmolality; freezing-point osmometers and vapor pressure osmometers. Because EG is nonvolatile (boiling point, 197° C), it is detected by either the freezing-point or vapor pressure methods. However, methanol, ethanol, and other volatile compounds, although contributing to serum osmolality, may go undetected if assayed by the vapor pressure method. Most clinical laboratories use the freezing-point method.70 Osmolality can be measured using serum or plasma; if the latter is used, heparin is the preferred anticoagulant. Other anticoagulants, such as ethylenediaminetetraacetic acid (EDTA), can markedly increase osmolality and can result in spurious increases in the osmole gap.70
Other procedures
Another diagnostic procedure that may be helpful in detecting early EG intoxication is examination of the oral cavity, face, paws, vomitus, and urine with a Wood’s lamp to determine whether they appear fluorescent. Many antifreeze solutions manufactured today contain sodium fluorescein, a fluorescent dye that aids in the detection of leaks in vehicle coolant systems. The dye is excreted in the urine for up to 6 hours following ingestion of the antifreeze.71 A negative test result does not eliminate the possibility of EG ingestion since not all antifreeze solutions contain the dye.
Ultrasonographic patterns in the kidneys of EG-intoxicated dogs and cats may be helpful in diagnosing late EG poisoning. A pattern of greater than normal cortical and medullary echogenicity with persistence of areas of lesser echo intensity at the corticomedullary junction and central medullary regions has been observed concurrent with the onset of clinical anuria and is referred to as the halo sign.72 Histopathological examination of kidneys taken at biopsy or necropsy from EG-poisoned animals is usually confirmatory because the renal tubules contain calcium oxalate crystals.
TREATMENT
Treatment should be instituted before confirmatory tests because EG is metabolized so quickly. Therapy for EG poisoning is aimed at preventing absorption, increasing excretion, and preventing metabolism of EG. Supportive care to correct fluid, acid-base, and electrolyte imbalances is also helpful. Although therapeutic recommendations have traditionally included induction of vomiting, gastric lavage, and administration of activated charcoal,73,74 it is likely that these procedures are not beneficial because of the rapidity with which EG is absorbed.31 Moreover, activated charcoal may not be of benefit because large quantities of charcoal are necessary to bind small amounts of EG.75 Absorption of ethanol is, however, notably inhibited by charcoal. Thus when oral ethanol is used as an emergency antidote, activated charcoal should definitely not be given.76
The most critical aspect of therapy is based on prevention of EG oxidation by ADH, which is the enzyme responsible for the initial reaction in the EG metabolic pathway.19 Historically, treating EG toxicosis has been directed toward inhibiting EG metabolism with ethanol, which is a competitive substrate that has a higher affinity for ADH than EG.77 Ethanol was first described as an effective antidote for EG intoxication in human beings in 1965 and was the therapy of choice for several years.78–80 Ethanol therapy was described in dogs and cats in the early to middle 1970s.16,81–83 Ethanol has numerous disadvantages because it enhances many of the metabolic effects of EG. Both ethanol and EG are CNS depressants, and it is the compounded CNS depression that most limits the usefulness of ethanol as an antidote. The CNS depression produced by high serum ethanol concentrations usually mandates intravenous (IV) fluid therapy for at least 48 hours. Moreover, ethanol, because of its short half-life as a competitive substrate of ADH,78,82,84 must be administered every 4 hours (IV) or, preferably, as a continuous IV drip, which often results in continuous intensive patient care. One suggested regimen to treat intoxicated dogs is to give 5.5 mL of 20% ethanol/kg body weight IV every 4 hours for five treatments and then every 6 hours for four additional treatments to maintain a serum ethanol concentration of approximately 50 to 100 mg/dL.85 A lower dosage is suggested for cats: 5 mL of 20% ethanol/kg body weight IV every 6 hours for five treatments and then every 8 hours for four additional treatments.73,85 Maintenance of more consistent serum levels of ethanol may be safer and more effective; a lower dose of 1.3 mL/kg of a 30% ethanol solution given as a bolus, followed by a constant IV infusion of 0.42 mL/kg/hr for 48 hours, has been shown to be as effective in preventing EG metabolism.86 Serum ethanol concentrations as low as 50 mg/dL (11 mmol/L) saturate ADH.80 Older references suggest intraperitoneal (IP) administration of ethanol to cats83; however, ethanol is irritating to the peritoneum, and this route of administration offers no advantages over IV drip administration. If pure ethanol for IV administration is unavailable, ethanol can be given orally, but gastric irritation may result in vomiting. An effective dose is approximately 2 to 3 mL/lb of an 80 proof (40% ethanol) alcoholic beverage.
Bolus injections, whether IV or IP, may increase serum ethanol concentrations to the point of suppressing respiration. In a study in which cats were experimentally poisoned with EG and then treated with IP ethanol at a dosage of 5 mL of 20% ethanol/kg of body weight every 6 hours, serum ethanol concentrations ranged from as low as 16 mg/dL at 6 hours after IP ethanol to as high as 240 mg/dL 30 minutes after IP ethanol. Cats with serum ethanol concentrations of more than 200 mg/dL appeared to be near respiratory arrest and were hypothermic.87 Although severe respiratory depression and coma usually develop when the serum ethanol concentration is 400 to 500 mg/dL, death caused by respiratory arrest has been reported in a human with a serum ethanol concentration of 260 mg/dL.88 Concentrations of 600 to 800 mg/dL in humans are almost always fatal.89
Additional disadvantages of ethanol treatment include its metabolism to acetaldehyde, which impairs glucose metabolism and is a cerebral irritant. Ethanol also contributes to metabolic acidosis by enhancing the formation of lactic acid from pyruvate90 and may potentiate hypocalcemia.91 Moreover, ethanol compounds the effects of EG-induced osmotic diuresis and serum hyperosmolality because, like EG, it is a small, molecular weight substance.70 The recommended therapeutic serum concentration of ethanol, 100 mg/dL (22 mmol/L), contributes 22 mosm/kg of H2O to the osmole gap.29 Another substrate with a high affinity for ADH, 1,3-butanediol, has been used experimentally in dogs and rats to prevent the metabolism of EG and has some advantages over ethanol.64a,92 However, it also contributes to the hyperosmolality, and metabolites of 1,3-butanediol contribute to metabolic acidosis.64a Despite its disadvantages, until recently ethanol has remained the therapy of choice in cats.54,93
4-Methyl-1H-pyrazole (fomepizole64a) has become the preferred antidote in dogs and more recently cats.* Fomepizole is an ADH inhibitor, not a competitive substrate, and it does not induce CNS depression (in dogs), diuresis, or hyperosmolality at the recommended dose. The parent compound, pyrazole, was reported to inhibit ADH in 1963.94 Fomepizole is a more potent inhibitor of ADH than pyrazole95 and has none of the toxic effects of pyrazole at recommended doses.†In a study in which the effectiveness of fomepizole therapy was compared with that of ethanol, dogs treated with fomepizole 3 hours after EG ingestion were clinically normal within 24 hours, whereas ethanol-treated dogs remained recumbent for 36 hours and were still severely depressed 72 hours after ingestion. Fomepizole-treated dogs continued to drink water, whereas ethanol-treated dogs were unable to drink, thus necessitating IV fluid therapy.38 When fomepizole was given to dogs as early as 3 hours following EG ingestion, approximately 90% of EG was excreted unmetabolized38,43,64a compared with approximately 80% when ethanol was used.38,64a Since 1982 approximately 160 dogs examined at Colorado State University’s Veterinary Teaching Hospital for suggested or confirmed EG intoxication have been treated with fomepizole.24,26 Adverse clinical signs were associated with the administration of fomepizole in only one dog. Tachypnea, gagging, excess salivation, and trembling developed after the second dose was given.26
The recommended dosage of fomepizole for dogs is 20 mg/kg of body weight IV initially, followed by 15 mg/kg IV at 12 and 24 hours, and 5 mg/kg IV at 36 hours.26,73,85 For humans receiving concurrent hemodialysis, it is suggested that a continuous infusion of 1 to 1.5 mg/kg/hr of fomepizole be given during the hemodialysis procedure because the drug is lost in the dialysate.98,99 Presumably, additional fomepizole should be given to dogs if peritoneal dialysis is used as concurrent therapy. The blood concentration of fomepizole can be determined by a high-performance liquid chromatographic assay.100
The efficacy of fomepizole treatment in cats has been evaluated using the dose for dogs.54 Fomepizole was not toxic in cats at this dose, and it inhibited ADH activity as evidenced by blocked EG metabolism when administered simultaneously with EG. However, fomepizole at this dose did not prevent development of renal failure when given 2 or 3 hours following EG ingestion, unlike ethanol, which prevented development of renal failure in four of six cats when given 3 hours after EG ingestion.54 Results of an in vitro study indicated that canine ADH was more effectively inhibited by fomepizole than feline ADH and suggested that higher doses of fomepizole might be more effective.101 A pilot study was conducted in which EG poisoning was induced in three cats by giving 1.7 g/kg of EG orally. The three cats were initially treated with 125 mg/kg of fomepizole IV at 1, 2, or 3 hours after EG ingestion. This dosage was followed by 31.25 mg/kg IV of fomepizole at 12, 24, and 36 hours. Constant IV fluid therapy was administered following the initial fomepizole treatment. Renal dysfunction did not develop in any of the cats, suggesting that the higher dose of fomepizole may be as effective as ethanol. The only adverse clinical sign observed in these cats was mild CNS depression, which appeared to be fomepizole related. In a larger study, eight adult cats were divided into two groups. One group of cats received fomepizole as therapy for ingestion of a lethal dose of EG using the above dose regimen from the pilot study. The other group of cats received ethanol using the bolus doses of 5 mL of 20% ethanol/kg at nine or more different time points.93 One cat from each group was treated 4 hours after EG ingestion; acute renal failure developed in both cats, and they required euthanasia. The remaining six cats were treated 3 hours after ingestion of a lethal dose of EG. All three of the fomepizole-treated cats survived; only one of the three ethanol-treated cats survived. Acute renal failure developed in one of the three cats treated with fomepizole; that cat was successfully treated with long-term IV fluid therapy and recovered (azotemia resolved but isosthenuria persisted). Acute renal failure developed in two of the three cats treated with ethanol. Because of severe acidemia that was nonresponsive to appropriate therapy, ongoing vomiting, severe CNS depression, hypothermia, and the onset of oliguric renal failure, those cats were euthanized.93 Results of that study suggested that high-dose fomepizole therapy was an effective and safe therapy for cats ingesting a lethal dose of EG, and the authors would recommend high-dose fomepizole therapy over ethanol to treat intoxicated cats. If ingestion of a large dose of EG is suspected, repeating serum quantification tests (via a reference laboratory or human hospital) would be advantageous to determine whether continuation of therapy beyond 36 hours is necessary. Alternatively, additional doses of fomepizole can be administered empirically.
Although the advantages of fomepizole therapy in dogs have been recognized since the early 1980s,64a lack of approval by the Food and Drug Administration (FDA) limited its use in animals because veterinarians were required to obtain an investigational new animal drug (INAD) number from the FDA before purchasing the drug from a chemical supplier. An additional inconvenience was the necessity to prepare a filtered 5% solution of the drug. Fomepizole has now been approved for veterinary use as an antidote for suggested or confirmed EG intoxication and is commercially available as Antizol-Vet,*which can be conveniently reconstituted. Fomepizole is also considered the therapy of choice for EG poisoning in human beings.36,102–111 Finally, metabolism of glyoxylic acid to nontoxic end products may be enhanced by the administration of thiamine and pyridoxine.112,113
Appropriate supportive therapy consists of IV fluids to correct dehydration, increase tissue perfusion, and promote diuresis. The fluid volume administered should be based on the maintenance, deficit, and continuing loss needs of the patient (Box 45-1). Frequent measurement of urine production, serum urea nitrogen and creatinine, and blood pH, bicarbonate, ionized calcium, and electrolytes daily or twice daily will help guide fluid and electrolyte therapy.114 Bicarbonate should be given slowly IV to correct the metabolic acidosis. (See Box 45-1 for replacement recommendation.) Ionized calcium should also be monitored closely, and calcium gluconate or calcium chloride can be supplemented as necessary (Box 45-1).