CHAPTER 17 Equine Rotavirus
Diarrhea is one of the most common medical problems of newborn foals, and rotavirus is the most common cause of foal enteritis in the major breeding centers of Kentucky, Ireland, and England.1,2 Both single cases of rotaviral diarrhea and severe farm outbreaks can occur. However, with the use of a commercially available vaccine, practical farm management practices, and hygiene measures, this disease can be controlled.
ETIOLOGY
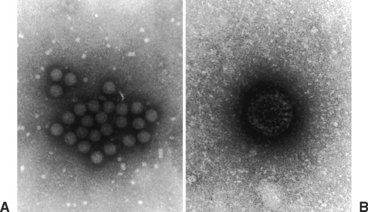
The family Reoviridae consists of five genera: Orthoreovirus, Orbivirus, Coltivirus, Aquareovirus, and Rotavirus. These are all double-stranded, ribonucleic acid (RNA), nonenveloped viruses with a diameter of about 80 nm (Fig. 17-1). The genus Rotavirus is subdivided into several groups (A-G) based on differences in the group-specific inner capsid protein, VP6. Equine and other animal isolates are in group A; groups B to G cause disease in humans, swine, fowl, and other animals.3 Further subdivision is made based on serologic assays using neutralizing antibodies to two outer capsid proteins, VP4 and VP7. Strains containing VP4 are referred to as P (protease-sensitive) serotype and strains containing VP7 as the G (glycoprotein) serotype.4 Serotypes G3, G5, G8, G10, G13, and G14 and serotypes P7, P12, and P18 have been described in horses.4–6 The G3 serotype has been identified in Kentucky and Japan and has been used in equine vaccine trials.7–9
Rotaviruses are stable within a pH range of 3 to 7 and are resistant to iodophor, quaternary ammonium, chlorine, and hypochlorite (bleach) disinfectants. Ethanol, phenols, and formalin can inactivate the virus.10–12
EPIDEMIOLOGY
Equine rotaviruses have been detected from diarrheic foals in the United States, Argentina, Britain, Ireland, Germany, Australia, New Zealand, and Japan. Equine rotaviruses only affect foals and are considered species specific; for example, cattle rotavirus affects cattle and not horses or humans. No natural reservoir for equine rotavirus has been identified. Mares do not shed the virus,1 except under experimental conditions.13 However, pregnant sows can shed the virus in feces from 5 days before to 14 days after farrowing, thereby seeding the environment for piglets.14 Considering the large concentrations of virus shed into the environment (1011 particles/g feces) by diarrheic animals, as well as the ability of the virus to remain viable for as long as 9 months,15 the potential for an outbreak after the first clinical case is very real.
Rotavirus is transmitted by the fecal-oral route through contaminated feces or fomites and is highly contagious. The incubation period is 12 to 24 hours. Adult horses are not clinically affected during outbreaks of foal diarrhea, but some mares with diarrheic foals will seroconvert, indicating subclinical infection.13 Studies of more than 400 adult horses performed in conjunction with rotavirus vaccination trials in Kentucky, Japan, and Argentina revealed a seroprevalence rate in broodmares approaching 100%.7–9 However, these studies were conducted in concentrated horse-breeding regions, and seroprevalence in adult horses in other geographic areas may be less.
The average age of foals with rotaviral diarrhea is 75 days, with a reported range of 2 to 155 days.1 The average duration of diarrhea in affected foals is 3 days (range, 1-9 days), with fecal shedding of rotavirus particles continuing for an average of 3 days after return of normal feces.
With appropriate supportive care, including fluid and electrolyte replacement therapy, rotaviral diarrhea has low mortality and high morbidity in foal populations. During a 3-year study of rotavirus on multiple central Kentucky horse farms, no foal had a confirmed recurrence of disease after recovery. In one study, fecal shedding of virus was demonstrated in clinically normal foals on 7 of 10 farms undergoing an outbreak of foal rotaviral diarrhea.1,16 This shedding does not continue after resolution of disease in other foals on the farm.
PATHOGENESIS
After rotavirus enters the gastrointestinal tract, it invades and rapidly multiplies in columnar epithelial cells of the villous tips in the duodenum and jejunum.17 This results in significant blunting of the villi and subsequent villous atrophy. The loss of these epithelial cells results in the absence of disaccharidases, especially lactase, causing a hyperosmotic solution in the intestinal lumen. This leads to malabsorption and maldigestion of nutrients and acute diarrhea. Intestinal crypt cells are not affected as they are in canine parvovirus infection. Therefore, crypt cells continue to replicate, differentiate, and eventually replace the tip cells destroyed by the virus, resulting in a self-limiting disease. Chronic diarrhea (>14 days) is not typical of rotavirus infection.
CLINICAL FINDINGS
The severity of rotavirus infection varies depending on the foal’s age and immune status, the virulence of the virus, and the quantity of viral inoculum. Approximately 18 to 24 hours after ingestion of infective material, foals show signs of lethargy, decreased suckling, and diarrhea that may vary from “cow pie” to watery consistency. With watery diarrhea, the foals’ tails may not be wet or stained with feces because of the projectile nature of the diarrhea. Fever may or may not be present. Younger foals are often more susceptible to severe disease because of their limited ability to self-correct fluid and electrolyte imbalances that accompany severe diarrhea (Fig. 17-2). Electrolyte imbalances may include hypochloremia, hyponatremia, hypokalemia, and acidosis. The hemogram is often normal or reveals evidence of hemoconcentration (e.g., increased packed cell volume).18