Nicholas Frank
Equine Metabolic Syndrome
Equine metabolic syndrome (EMS) is a collection of endocrine and metabolic abnormalities associated with the development of laminitis in equids. The American College of Veterinary Internal Medicine consensus statement on EMS published in 2010 lists three major components: increased adiposity in specific locations (regional adiposity) or generally (obesity), insulin resistance (IR) with hyperinsulinemia, and a predisposition to development of laminitis. However, laminitis is a consequence of the syndrome and can be avoided if management changes are made. The current definition of EMS focuses on hyperinsulinemia and also includes IR, increased adiposity, hyperleptinemia, and hypertriglyceridemia. The term insulin dysregulation has been introduced to encompass the problems of increased insulin secretion, decreased hepatic insulin clearance, and peripheral IR.
Alternative names for EMS include insulin resistance syndrome, peripheral Cushing’s syndrome, and prelaminitic metabolic syndrome. Clinical signs of EMS were attributed to hypothyroidism in the past, but the low resting thyroid hormone concentrations detected in some horses with EMS are better thought of as a consequence, rather than a cause, of obesity.
Clinical Presentation
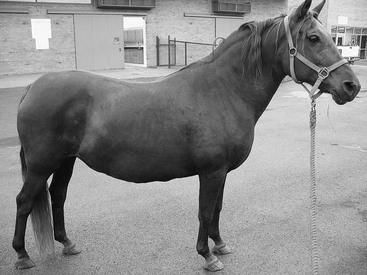
Equine metabolic syndrome can affect all domesticated equids. Pony, Morgan Horses, Paso Finos, and Norwegian Fjord breeds are overrepresented, but the syndrome is recognized in many other breeds of horse, including Arabians, Quarter Horses, Saddlebreds, Tennessee Walking Horses, and warmbloods. Horses are mature when EMS is first recognized, and the age of onset for laminitis is determined by the conditions under which the horse has been kept. Genetically predisposed horses that are allowed to become obese and graze on pasture with abundant grass can develop laminitis at a young age, whereas other susceptible horses that are managed appropriately can avoid laminitis altogether. Divergent growth rings, also called founder lines, are sometimes present on the hoof wall, indicating previous episodes of subclinical laminitis. These protruding growth rings are closer to the coronary band dorsally than they are at the heel, and are thought to occur when laminitis inhibits dorsal hoof wall growth. Physical characteristics of EMS include generalized obesity, regional adiposity, or both. Regional adiposity takes the form of cresty neck in horses, and neck circumference has been negatively correlated with insulin sensitivity. Other manifestations of regional adiposity include abnormal adipose tissue deposits close to the tailhead, within the prepuce, or randomly distributed beneath the skin (Figure 135-1).
Many horses are on pasture when laminitis first develops, and episodes are more common in the spring and fall. Other horses with EMS are first recognized when they present with infertility problems. Colic caused by pedunculated lipomas is a concern in obese equids.
Pathophysiology
It has long been recognized that EMS is more common in certain breeds of horse, and research is presently being conducted to examine the genetic basis of this syndrome. If it is assumed that some horses are genetically predisposed to EMS, diet and exercise are two important modifying factors that can affect expression of the phenotype. A genetically predisposed horse that is overfed is more likely to express the EMS phenotype, whereas the same horse might remain healthy if kept in a lean condition, fed appropriately, and exercised regularly. Obesity is an important modifying factor because it can induce IR, and this raises insulin concentrations. The concept of pathologic fat must also be considered because adipose tissues secrete proinflammatory cytokines as obesity develops. This is accompanied by a shift in adipokine production, with increased leptin secretion and decreased adiponectin production. Detection of high leptin concentrations confirms that changes in fat metabolism have occurred, and hyperleptinemia is associated with insulin dysregulation. Hyperinsulinemia and hyperleptinemia might contribute to the so-called easy keeper metabolic state recognized in horses with EMS.
Insulin dysregulation is a key component of EMS, and postprandial hyperinsulinemia may explain why affected horses grazing on pasture develop laminitis. Laminitis has been experimentally induced in both ponies and Standardbred horses by infusing insulin intravenously at high levels. Postprandial hyperinsulinemia, fasting hyperinsulinemia, and tissue IR occur to varying degrees in horses with EMS, but temporal relationships among these factors require further investigation. One hypothesis is that genetically predisposed horses first develop postprandial hyperinsulinemia, and then IR develops over time as the condition progresses. Fasting hyperinsulinemia is the last abnormality to develop as fatty acids stimulate insulin secretion or beta cell hyperplasia develops. Postprandial hyperinsulinemia is a plausible starting point for horses with EMS because high insulin concentrations can induce IR through a process of homologous desensitization. This phenomenon is recognized when an insulinoma develops and secretes excessive amounts of insulin. Hyperinsulinemia-induced IR is also a potential mechanism for insulin-induced laminitis. Insulin resistance within endothelial cells would be expected to promote vasoconstriction, alter normal blood flow dynamics, and reduce nutrient delivery to laminar tissues.
Hyperinsulinemia can develop as a consequence of increased secretion from pancreatic beta cells or slowed clearance from the blood. Increased insulin secretion following meals might be attributed to altered regulation of incretin hormones; these include glucagon-like peptide 1 and gastrointestinal polypeptide, which are secreted from the small intestine in response to ingested sugars and other nutrients and stimulate the insulin secretion from pancreatic beta cells. Incretin hormones stimulate insulin secretion and slow gastric emptying as glucose concentrations rise after feeding, and this minimizes postprandial hyperglycemia. Both incretin hormones are degraded by the enzyme dipeptidyl transferase-4, so postprandial hyperinsulinemia might result from increased secretion of incretin hormones or slowed degradation. Alterations in incretin hormones could explain the development of postprandial hyperinsulinemia and laminitis in equids grazing on pastures that are rich in simple sugars, starch, and protein.
Diagnostic Testing
Diagnostic tests for EMS are summarized (Table 135-1). Recommendations for diagnostic testing have shifted recently as greater emphasis has been placed on oral glucose testing. Because insulin concentrations increase after feeding, postprandial hyperinsulinemia is a major concern, particularly when horses are consuming grass on pasture. Equids that are genetically susceptible to postprandial hyperinsulinemia might also be predisposed to obesity and have a higher risk for laminitis. Previous recommendations focused on fasting insulin concentrations and the combined glucose-insulin test (CGIT). Both remain useful tests and are still recommended for diagnosing IR, but they do not assess the insulin response to ingested sugars, which may be the first manifestation of insulin dysregulation in equids. A CGIT is performed by infusing 50% dextrose solution, immediately followed by intravenous administration of regular insulin, so incretin hormone responses are bypassed. This dynamic test determines the rate of glucose disposal into tissues, which is a measure of insulin sensitivity and the overall insulin response to glucose. The insulin response is also assessed and reflects the rate of insulin secretion from pancreatic beta cells and insulin clearance rate, but does not account for insulin release stimulated by incretin hormones after feeding.
TABLE 135-1
Recommended Diagnostic Tests for Equine Metabolic Syndrome
| Test | Procedure | Interpretation* |
| Endocrine and metabolic status panel | ||
| Glucose Insulin Triglycerides Leptin ACTH† | Fasting required. Leave only one flake of hay in the stall after 10 pm the night before and collect blood in the morning. Collect blood into one EDTA and one serum tube. | Persistent hyperglycemia indicates diabetes mellitus (insulin is normal or increased). Hyperinsulinemia if fasting insulin concentration >20 µU/mL (mU/L). Hypertriglyceridemia if >50 mg/dL; concern if >27 mg/dL. Hyperleptinemia if leptin concentration >4 ng/mL. Refer to PPID (see Chapter 136) for ACTH interpretation. |
| Oral sugar test (OST) | ||
| This test is recommended to assess the combined effects of incretin hormones, pancreatic beta cell insulin secretion, and insulin resistance on insulin concentrations. If the owner has concerns about inducing laminitis, a two-step approach is recommended. First, measure fasting insulin concentrations. If within reference range, proceed to the OST. | Fasting required (see above) Owner administers 0.15 mL per kg (approximately 75 mL) Karo Light‡ corn syrup orally with 60-mL catheter-tip syringes. Collect blood 60 and 90 min after administration of corn syrup. Measure glucose and insulin concentrations. | Normal if the insulin concentration is <45 µU/mL at 60 and 90 min. Hyperinsulinemia if the insulin concentration is >60 µU/mL at 60 or 90 min. Equivocal result if the insulin concentration is 45 to 60 µU/mL at 60 or 90 min. Repeat testing at a later time or consider other tests. Excessive glucose response if the glucose concentration is >125 mg/dL at 60 or 90 min. |
| Test meal | ||
| Insulin concentrations can be measured after feeding to assess postprandial hyperinsulinemia. | Owner feeds the horse as normal and contacts the veterinarian when the meal has been consumed. Veterinarian collects two blood samples 30 min apart in the period 90 to 150 min following the meal. | Reference ranges have not been established. |
| Insulin tolerance test (ITT) | ||
| Hyperinsulinemia can be caused by increased insulin secretion from the pancreatic beta cells and/or insulin resistance. The ITT is used to detect tissue insulin resistance. | Fasting required (see above) Step 1: Collect a baseline blood sample and inject regular (soluble) insulin§ intravenously at a dosage of 30 mU/kg (0.03 µU/kg). Then collect a second blood sample 30 min after injection. Proceed to step 2 if the glucose concentration has not decreased by 50%. Step 2: Repeat the test on a different day and administer insulin at a dosage of 100 mU/kg (0.10 µU/kg). Feed as normal after the second blood sample. | Horses with normal insulin sensitivity have a 50% decrease within 30 min in response to the 100 mU/kg dose of insulin. If this response is observed with the lower 30 mU/kg dosage, no further testing is required. Hypoglycemia is a concern with this test, and dextrose solution should be kept on hand. |
* Cutoff values for assays performed by the Animal Health Diagnostic Laboratory at Cornell University, Ithaca, NY. Insulin and leptin measured by radioimmunoassay and ACTH by chemiluminescent assay.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree