Gisela Soboll Hussey, Gabriele A. Landolt
Equine Alphaherpesviruses
Herpesviruses are large double-stranded DNA viruses that are ubiquitous pathogens affecting most mammals, including horses. The subfamily of Alphaherpesvirinae in horses includes equine herpesvirus-1 (EHV-1), equine herpesvirus-4 (EHV-4), and equine herpesvirus-3 (EHV-3). Infection with EHV-1 and EHV-4 is one of the most common causes of viral respiratory disease in horses worldwide, and estimates of prevalence show that most adult horses are infected with EHV-1, EHV-4, or both throughout their lifespan. EHV-1 also significantly affects the equine industry by causing late-term abortions, equine herpesvirus myeloencephalopathy (EHM), and chorioretinopathy. Although EHV-4 is capable of causing secondary disease, similar to EHV-1, this occurs much less commonly than is observed with EHV-1. Infection with EHV-3 is the cause of equine coital exanthema and venereal disease in equine breeding populations.
The most critical epidemiologic feature of alphaherpesvirus infection is that most horses are infected early in life, with low morbidity and establishment of lifelong latency in up to 70% of infected horses. This feature, along with frequent viral reactivation, ensures survival of the virus in horse populations. In addition, viral survival depends on a number of immune-evasive and immune-suppressive mechanisms that are initiated by the virus and prevent the host’s immune system from establishing long-term protective immunity. Immunity following natural infection or vaccination is thus typically brief, and control of EHV by vaccination remains problematic.
Pathogenesis
Equine Herpesvirus-1 and Equine Herpesvirus-4
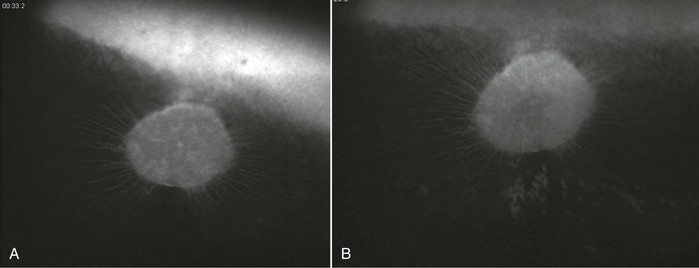
Infection with EHV-1 and EHV-4 occurs through the respiratory tract by inhalation of aerosolized infectious virus, nose-to-nose contact, or contact with fomites. Following infection, the virus replicates in the respiratory airway epithelium and causes erosion of the respiratory mucosa and viral shedding through nasal secretions into the environment. The virus spreads quickly to the cells of the underlying tissues and can typically be detected in local lymph nodes of the respiratory tract within 24 to 48 hours after infection. Although infection with EHV-4 is mostly limited to the respiratory tract and local lymphoid tissues, for EHV-1, a cell-associated viremia is established between days 4 and 10 after infection, and the virus is transported to sites of secondary infection, where contact with the vascular endothelium allows for endothelial cell infection, inflammation, thrombosis and tissue necrosis, and secondary disease manifestations directly following viremia on days 9 to 13 after infection. A positive correlation between the duration and magnitude of viremia and incidence of EHM has been identified, but whereas EHM is unlikely to develop in the absence of viremia, only a small percentage (about 10%) of viremic horses subsequently develop EHM. Other host and viral factors associated with development of EHM include age, breed, sex, and the presence of a single nucleotide polymorphism in the viral polymerase gene that results in an amino acid change at position (N752 vs. D752), with D752 being strongly associated with neuropathogenicity. Interestingly, the incidence of late-term abortions caused by EHV-1 is higher (about 50%) than the incidence of EHM. This may be related to the hormonal milieu and altered immune system in the last trimester of pregnancy. The pathogenesis of viral invasion at the vascular endothelium of the pregnant uterus is, however, similar to the pathogenesis of EHM, with viremia precipitating infection of endothelial cells of the endometrium leading to vasculitis, thrombosis, microcotyledonary infarction, perivascular cuffing, and transplacental spread of virus at the sites of vascular lesions. EHV-1 chorioretinopathies are less significant economically and clinically because most ocular infections are subclinical and rarely lead to loss of function or even immediate clinical signs. The incidence of ocular lesions can be higher than 50% following experimental and natural infection, and ocular pathology is typically caused by a vascular endotheliopathy, with subsequent ischemic injury to the chorioretina resulting from viremia and direct infection of the vascular endothelium. Because the choroidal vasculature is typically hidden by the pigmented retinal epithelium, ocular lesions are not usually visible in vivo until 1 month or later after infection (Figure 37-1). At the onset of EHM, in the more acute stages of infection, inflammatory change and viral antigen can only be detected postmortem by histologic evaluation of the choroidal vasculature.
Equine Herpesvirus-3
Infection with EHV-3 occurs through direct skin-to-skin contact during coitus or through secretions containing live virus (i.e., secretions on contaminated materials used during artificial insemination or on hands or even the lips and nose of horses nuzzling or sniffing one another). No disruption of the skin barrier is necessary to establish infection, and viral replication is limited to the stratified epithelium, leading to localized inflammation with development of the typical cutaneous lesions. Spread of the virus to the underlying tissues and dissemination through the bloodstream does not occur. Secondary bacterial infection is common and affects the severity and duration of clinical disease. Most horses clear the infection spontaneously within 2 to 3 weeks of infection, but the virus establishes latency in a high percentage of horses, and recrudescence of the virus and clinical signs can be observed in horses in consecutive breeding seasons.
Clinical Manifestations
Equine Herpesvirus-1 and Equine Herpesvirus-4
Clinical manifestations of EHV-1 infection include primary respiratory disease, late-term abortions, neonatal foal death, EHM, and chorioretinopathy. EHV-4 infections are usually restricted to the respiratory tract. Infection with EHV-1 or EHV-4 typically affects the upper respiratory tract, and can be mild or asymptomatic in older or previously exposed horses. In contrast, the respiratory disease observed in young immunologically naïve horses is often severe, lasts for 2 to 3 weeks, and is characterized by a biphasic fever, depression, anorexia, coughing, and nasal and ocular discharge that is initially serous and then becomes mucopurulent. Often horses develop lymphadenopathy of the respiratory tract lymph nodes that is accompanied by lymphopenia and neutropenia that lasts for several days. Lower respiratory tract disease associated with secondary bacterial infection, tachypnea, anorexia, and depression can be observed in young foals. EHV-1 and EHV-4 respiratory infections are difficult to distinguish clinically from other infections unless the virus is identified diagnostically.
Equine herpesvirus-1 is also the cause of late-term abortions and premature delivery of foals that die soon after birth. Mares infected with EHV-1 often appear healthy but abort 2 weeks to several months after infection or reactivation of the virus. Abortion typically occurs without warning in the last trimester of pregnancy, and the placenta is commonly found together with the fetus, which has died from asphyxia or dies shortly after birth. Sporadic abortions in individual mares are most common, but EHV-1 outbreaks with attack rates higher than 50% (so-called abortion storms) have been reported; the manifestation of infection on any given premises depends on herd management, immune status, and virus-related factors. Mares typically recover and deliver a healthy foal in the following breeding season. Occasionally, apparently healthy foals are delivered that become ill within 2 days of delivery and show respiratory distress, fever, failure to nurse, weakness, diarrhea, and leukopenia, and do not respond well to treatment. These foals were likely infected perinatally or during birth. EHV-1 can also be found in the sperm of infected stallions, but venereal transmission has not been described to date.
Equine herpesvirus myeloencephalopathy affects the central nervous system, and outbreaks are characterized by a large number of horses affected with mild to moderate respiratory disease and a fever, with 10% to 40% of infected horses developing EHM. Clinical signs appear after the onset of viremia and include ataxia and paralysis that can lead to recumbency and urinary incontinence, which often result in euthanasia (see Chapter 90 for more information on neurologic herpesvirus disease).
Equine herpesvirus-1 infection of the ocular endothelium leads to chorioretinopathy, which causes permanent “shotgun” lesions in a substantial proportion of infected horses; however, the connection between ocular lesions and EHV-1 infection is often not made. In a recent study, more than 50% of yearling horses developed classic shotgun ocular lesions following experimental infection with EHV-1, from 4 weeks to 3 months after primary infection (see Figure 37-1). Lesions can be focal, multifocal, or, rarely, diffuse, which affects the entire eye. Clinically, only diffuse lesions have a significant impact and cause loss of vision.
Equine Herpesvirus-3
The lesions of EHV-3 infection are restricted to the superficial skin of the external genitalia in both mares and stallions. Lesions typically start as small raised papules and progress from vesicle to pustule and finally to raw or encrusted erosion or ulcer on the vagina, penis, prepuce, and perineum and occasionally the lips and teats. In addition, local inflammation, characterized by redness and swelling, is commonly observed. Uncomplicated cases typically resolve within 10 to 14 days, but depigmentation and cutaneous scars persist longer. Occasionally, severely affected horses may be febrile and become depressed and inappetant. Stallions may show a loss of libido and refuse to mount, and mares may show frequent urination, with arching of the back and vulvar discharge. Severity and duration are also influenced by secondary bacterial infections, and secondary infection with Streptococcus zooepidemicus is common.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree