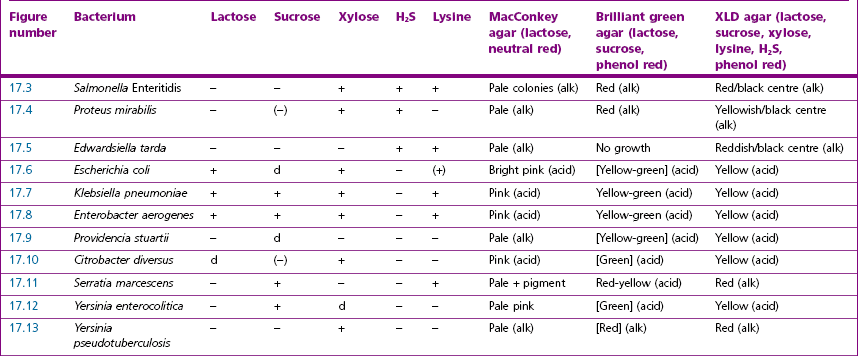
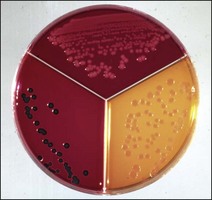
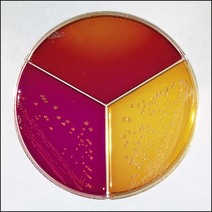
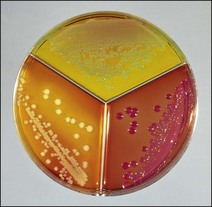
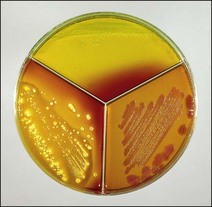
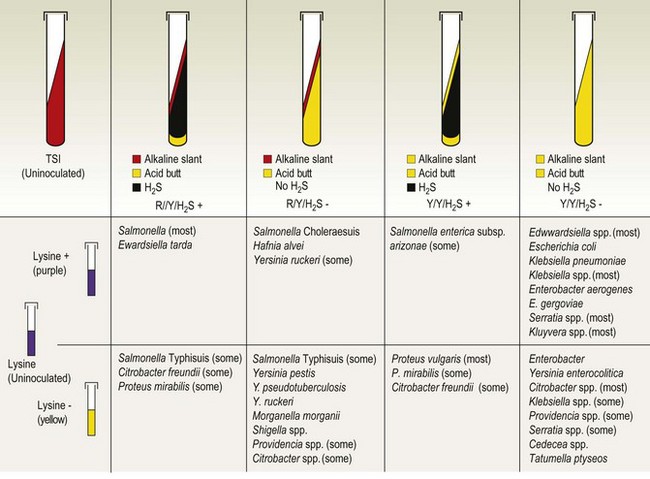
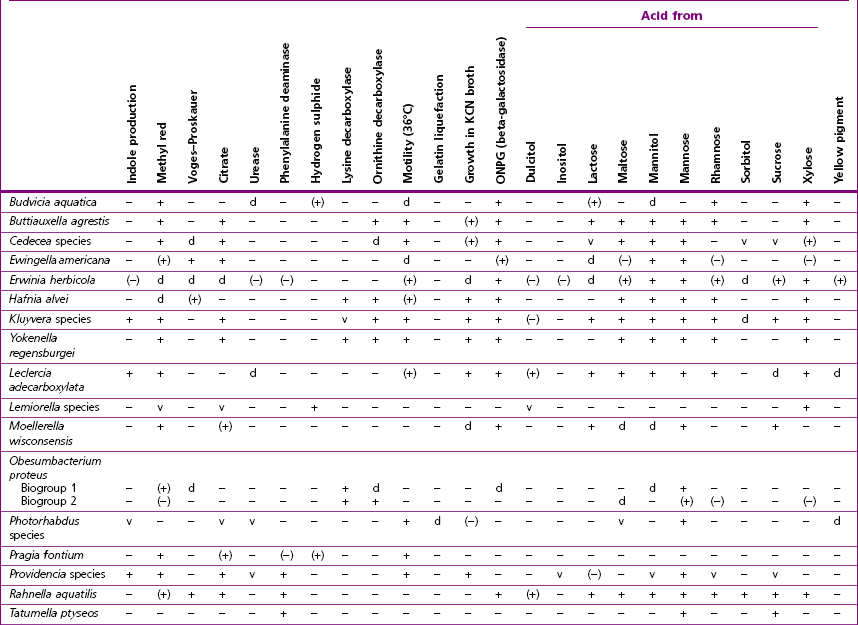
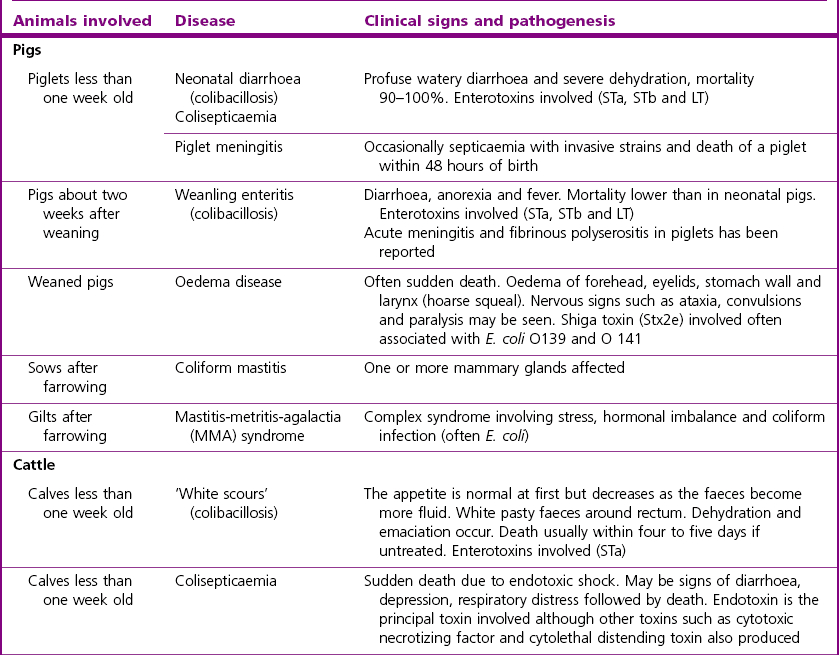
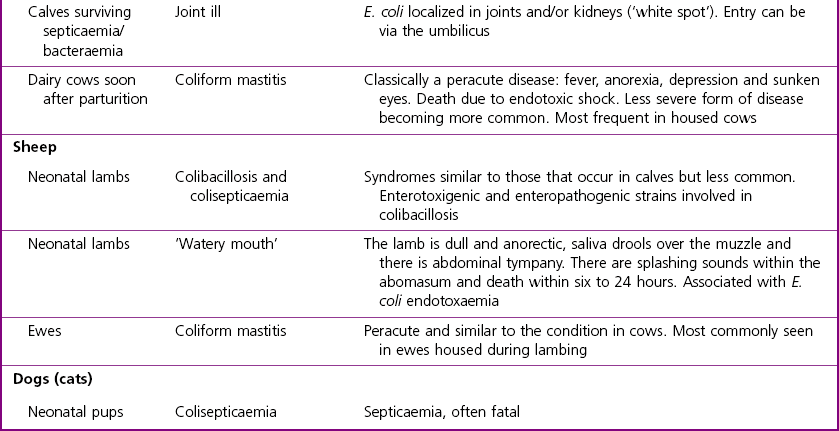
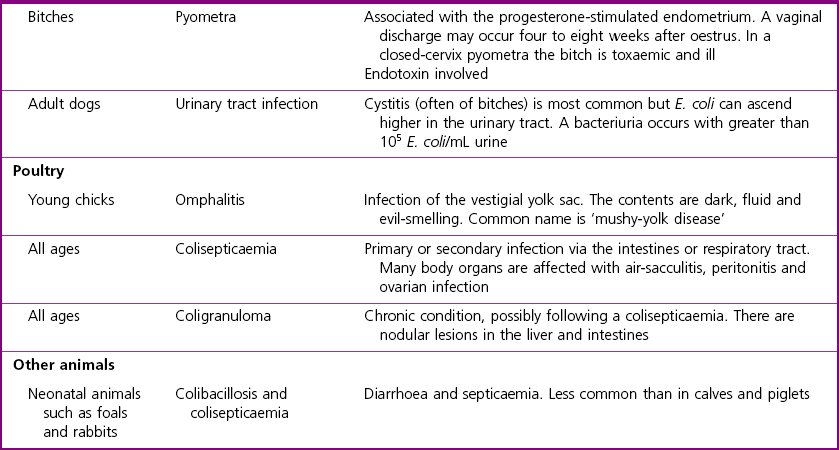
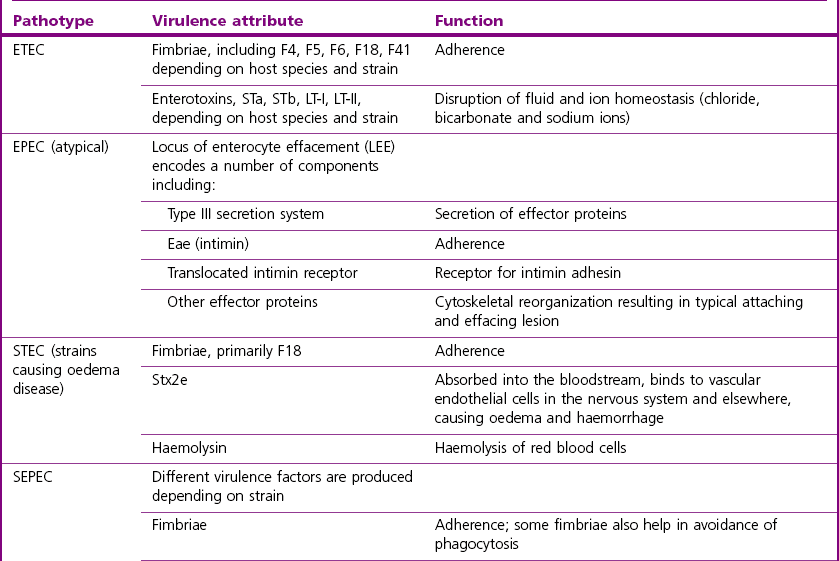
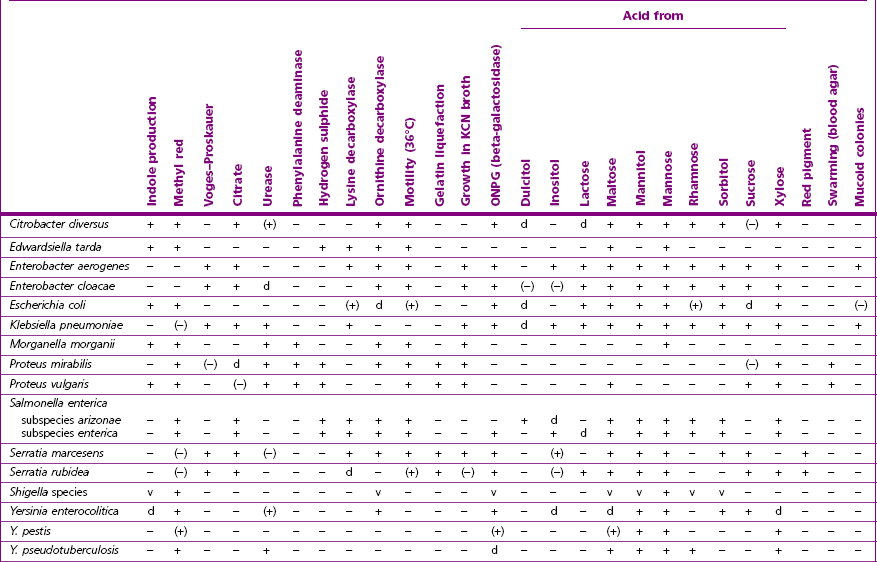
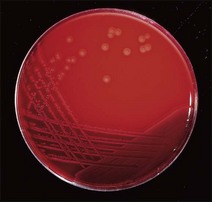
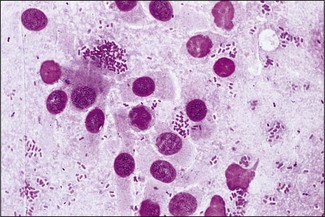
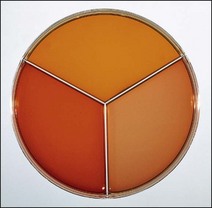
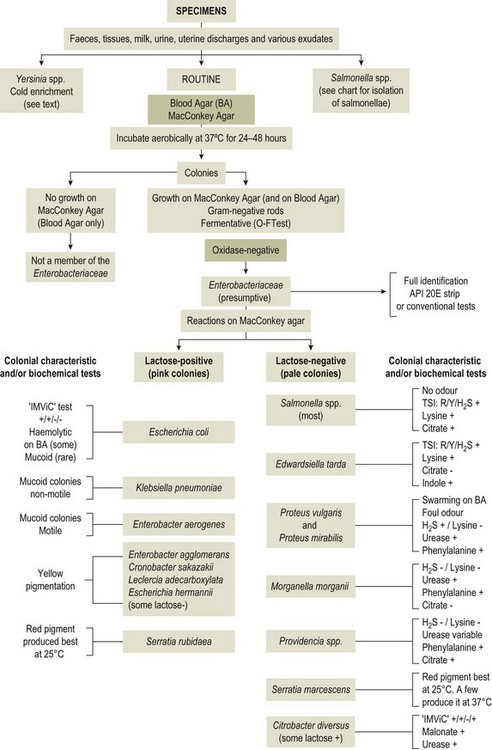
Chapter 17 Most members of the family Enterobacteriaceae share the following characteristics: Gram-negative, medium-sized rods (0.4–0.6 × 2–3 µm; Fig. 17.1); peritrichate arrangement of flagella, if motile; facultatively anaerobic and ferment, rather than oxidize, glucose; catalase-positive and oxidase-negative; reduce nitrate to nitrite and are able to grow on non-enriched media such as nutrient agar. There are a few exceptions to these general properties, for example, Shigella dysenteriae is catalase-negative; Tatumella ptyseos is motile by polar, subpolar or lateral flagella and Photorhabdus species do not regularly reduce nitrate. Figure 17.1 Gram-negative medium-sized rods of Escherichia coli in a tissue smear. The morphology is typical of most members of the Enterobacteriaceae. (Gram stain, ×1000) All enterobacteria will grow on blood and MacConkey agars and these are used routinely to isolate them in diagnostic laboratories. Although MacConkey agar is a selective medium, it is relatively permissive and allows the growth of some other Gram-negative bacteria as well as the enterobacteria. Brilliant green agar and xylose-lysine-deoxycholate (XLD) medium are more selective and used for the isolation of salmonellae, although some other enterobacteria are able to grow on them. A number of other selective agars are frequently used for the isolation of salmonellae and these are detailed under the relevant section. Table 17.1 gives the reactions of some members of the Enterobacteriaceae on MacConkey agar, brilliant green agar and XLD medium. The uninoculated media (Fig. 17.2) are illustrated together with the appearance of 11 enterobacteria and Pseudomonas aeruginosa on these selective/indicator media (Figs 17.3 to 17.14 inclusive). A summary of isolation methods for the detection and presumptive identification of important members of the Enterobacteriaceae is given in Figure 17.15. Figure 17.2 Uninoculated selective media used for the isolation of members of the Enterobacteriaceae: XLD medium (left), brilliant green agar (top) and MacConkey agar (right). Figure 17.14 Reactions of Pseudomonas aeruginosa on XLD medium (left), brilliant green agar (top) and MacConkey agar (right) for comparison with the reactions of the Enterobacteriaceae. Figure 17.15 Routine isolation of important members of the Enterobacteriaceae and their presumptive identification on colonial morphology and /or biochemical tests. pH indicator: neutral red (pale-straw at pH 8 and pink at pH 6.8). Inhibitors: bile salts and crystal violet (anti-Gram-positive bacteria). Reactions: if the bacterium can ferment lactose, acid metabolic products are produced and the medium and colonies appear pink (lactose-positive). If the organism is unable to use the lactose, then it attacks the peptone (nitrogen source) in the medium with resulting alkaline metabolic products and the medium and colonies appear pale/straw-coloured (lactose-negative). Fermentable sugars: lactose and sucrose pH indicator: phenol red (red at pH 8.2 and yellow at pH 6.4) Inhibitor: brilliant green dye inhibits the growth of most enterobacteria to some extent, except Salmonella species. Reactions: similar to those occurring on MacConkey agar except that the bacteria may ferment one or both of the sugars with an acid reaction (yellowish-green). Certain enterobacteria, such as Salmonella species, are unable to ferment either sugar and attack the peptone instead, with an alkaline reaction (red colonies and medium). Fermentable sugars: lactose, sucrose and xylose. pH indicator: phenol red (red at pH 8.2 and yellow at pH 6.4). Other substrates: lysine and chemicals for detecting hydrogen sulphide (H2S) production. Inhibitor: bile salts (sodium deoxycholate). Reactions: salmonellae will first ferment the xylose creating a temporary acid reaction but this is reversed by the subsequent decarboxylation of lysine with alkaline metabolic products. Superimposed on the red (alkaline) colonies is the production of hydrogen sulphide, so most salmonellae have red colonies with a black centre (Fig. 17.3). Edwardsiella tarda also gives this reaction although the H2S production is less marked and the periphery of the colonies tends to be a yellowish-red colour. The large amount of acid produced by enterobacteria that can ferment either lactose or sucrose, or both, prevents the reversion to alkaline conditions even if the bacterium is able to decarboxylate the lysine. This is an indicator medium only and does not contain an inhibitor. A brief description of the medium and technique for inoculation is given in Chapter 2. It is prepared in tubes with a slant. Fermentable sugars: glucose 0.1%, lactose 1.0% and sucrose (or saccharose) 1.0%. Other substrates: chemicals to indicate hydrogen sulphide (H2S) production. pH indicator: phenol red (red at pH 8.2 and yellow at pH 6.4). Reactions: all members of the Enterobacteriaceae are capable of fermenting glucose and the small amount (0.1%) will be attacked preferentially and rapidly. At this early stage both the butt and slant will be yellow due to acid production from the glucose fermentation. Some enterobacteria attack the lactose and/or sucrose (each at a 1.0% concentration) in the medium and in this case sufficient acid is produced to maintain both the butt and the slant in an acid (yellow) condition. Bacteria that are unable to ferment either lactose or sucrose, after the depletion of the limited amount of glucose, will use the peptone in the medium. This is a less efficient method of producing energy and occurs mainly at the surface of the slant in the presence of atmospheric oxygen. The metabolites of peptone are alkaline and this causes the slant to revert back to the original red colour. Some members of the Enterobacteriaceae, including most Salmonella spp., are able to produce hydrogen sulphide. This reaction is superimposed over the sugar fermentations and is seen as a blackening of the medium. The general interpretation of the reactions is as follows: • Alkaline (red) slant and acid (yellow) butt: glucose fermentation only. • Acid (yellow) slant and acid (yellow) butt: lactose and/or sucrose attacked as well as the glucose. The reactions are illustrated in Figure 17.16, while Figure 17.17 gives a summary of the differentiation of some of the enterobacteria by their reactions in TSI agar and lysine decarboxylase broth. Figure 17.16 TSI agar slopes showing the range of reactions from the left, uninoculated, R/Y/H2S+, R/Y/H2S−, Y/Y/H2S+, Y/Y/H2S−. The Enterobacteriaceae can be divided into three groups based on their pathogenicity for animals: • Major pathogens of animals such as Salmonella species, Escherichia coli and three of the Yersinia species. • Opportunistic pathogens that are known to occasionally cause infections in animals. These include species within the genera Klebsiella, Enterobacter, Proteus, Serratia, Edwardsiella, Citrobacter, Morganella and Shigella. Shigella species cause disease in humans and other primates. • Organisms of uncertain significance for animals. These include species from 17 genera of the Enterobacteriaceae and they are summarized in Table 17.2. As some of them may be isolated from clinical specimens a range of their biochemical reactions is given in Table 17.3. Table 17.2 Genera of the Enterobacteriaceae whose species are of uncertain significance for animals • Neonates obtaining insufficient passive immunity (antibodies) from colostrum. This might be due to either a quantitative or qualitative deficiency. • Intensive husbandry practices lend themselves to rapid transmission of the pathogenic E. coli strains. • Poor hygiene that allows a build-up of pathogenic strains in the environment of the young animal. A large dose of pathogenic E. coli may overcome colostral immunity. • Young neonates, under one week of age, are particularly susceptible because: • Recently weaned pigs are subject to stress factors such as altered surroundings, companions and diet. Heavy grain diets in particular can lead to a massive colonization of the anterior small intestine by enterotoxigenic strains of E. coli. • Oedema disease occurs most commonly in young weanling pigs but the disease can occur in older pigs. The following factors are often present prior to the occurrence of oedema disease in pigs: Escherichia coli strains can be divided into those causing extraintestinal disease and those causing enteric infections. Extraintestinal diseases result from infection with strains causing invasive conditions such as septicaemia (SEPEC), and also include uropathogenic E. coli (UPEC) and avian pathogenic E. coli (APEC). These strains may be collectively referred to as extraintestinal pathogenic E. coli or ExPEC. There are also suggestions for two new animal pathogenic groups: those causing infections of the mammary gland, mammary pathogenic E. coli (MPEC) and those affecting the uterus, endometrial pathogenic E. coli (EnPEC) (Köhler & Dobrindt, 2011). The types of E. coli causing enteric disease include enterotoxigenic strains (ETEC) and attaching and effacing E. coli (AEEC). The latter group includes the enteropathogenic E. coli (EPEC) and Vero- or Shiga-toxin producing strains (VTEC or STEC). Enterohaemorrhagic E. coli and strains of E coli producing oedema disease are subgroups of STEC. Of the disease syndromes caused by these pathogenic types, enteric disease produced by ETEC, oedema disease of pigs and extraintestinal diseases, including septicaemia, are the best characterized types in animals. In contrast to ETEC strains, ExPEC and EPEC strains form part of the normal flora in animals and are considered opportunistic pathogens (Gyles & Fairbrother, 2010). These strains cause the majority of cases of neonatal colibacillosis in calves, lambs and piglets. They do not appear to be an important cause of diarrhoea in other domestic animals, for reasons which are as yet unclear. Pathogenicity is correlated with the presence of adhesins and the production of enterotoxins. The toxins function by reducing absorption and increasing secretion without damaging the intestinal epithelium. The first step in the production of disease is the adherence of the ETEC to the intestinal epithelium. The structures by which the ETEC adhere are most commonly fimbriae and these are classified according to properties such as their amino acid composition and their ability to agglutinate red blood cells in the presence or absence of D-mannose. The fimbriae described in pigs and calves include F4 (K88), F5(K99), F6 (987P), F17, F18 and F41 (Nagy & Fekete 1999). ETEC strains are host-specific and usually cause disease in the first week of life only. Host specificity can be explained by the presence or absence of genes encoding for fimbrial receptors in the intestinal lining of the host. Age-related resistance may be due to the degree of expression of host receptors. It appears that some of the receptors are over-expressed as the age of the animal increases, thus leading to shedding of receptors into the intestinal lumen. These free receptors coat the ETEC strains in the intestinal contents, thus preventing their adherence to the intestinal lining (Dean et al. 1989). F18 receptors are not produced in newborn pigs but are increasingly expressed up to approximately four weeks of age, thus helping to explain why ETEC are a cause of diarrhoea in pigs up to this age. Following adherence, ETEC produce protein enterotoxins. The toxins can be subdivided into two main groups: Large heat-labile toxins of approximately 88 kDa in size (LT) and smaller, heat-stable toxins (ST) containing 11–48 amino acids. Both toxin types have two subgroups. Porcine strains of ETEC produce mostly LT1 and Sta whereas strains of ETEC occurring in calves usually produce Sta. STb is associated with porcine strains of ETEC although it may be produced by some strains of ETEC isolated from calves, chickens and humans. LT has a similar mechanism of action to the heat-labile toxin produced by Vibrio cholerae. It consists of an A domain and five B subunits. The B subunits bind to the cell and part of the A domain then enters the endoplasmic reticulum of the cell and activates the adenylate cyclase system. The resulting increase in cAMP levels leads to increased fluid and electrolyte secretion and decreased absorption. STa acts by increasing guanylate cyclase activity leading to elevated levels of cGMP in the cell. Increased levels of cAMP or cGMP activate protein kinases which induce phosphorylation of the cystic fibrosis transmembrane regulator (CFTR). This in turn causes secretion of chloride and bicarbonate ions. Protein kinases also inhibit reabsorption of sodium ions (Dubreuil 2012). There is a resultant reduction in absorption of water and electrolytes at the villus tips and an elevated secretion of chloride and water in crypt cells. The mechanism of action of STb differs from that of LT and STa as it does not activate adelyate or guanylate cyclases but phosphorylates CFTR through a different mechanism. Interference with the enteric nervous system may also be important in the secretory diarrhoea induced by E. coli enterotoxins (Dubreuil 2012). Septicaemic (SEPEC) strains are responsible for septicaemia in their hosts. These strains may possess a wide range of virulence factors but few of these virulence factors are common to all strains (Mokady et al. 2005). It appears that each step of the disease process can be mediated by a number of alternative virulence factors and a particular invasive strain may have a unique combination of pathogenic attributes. Adherence to the intestinal lining is the first step in the invasion process. Adherence may be mediated by fimbrial adhesins, for example, F5 as in ETEC strains, by other fimbriae such as long polar fimbriae or by non-fimbrial adhesins. Septicaemic strains carry a plasmid encoding Colicin V (Col V plasmid). This plasmid encodes for type IV pili which have been shown to be important for adherence and invasion in Salmonella Typhi. In addition it encodes for serum resistance and the aerobactin iron uptake system, both important virulence factors for systemic survival of E. coli strains. Recently the presence of another iron uptake system has been demonstrated, similar to that found in Yersinia species, and dependent on the biosynthesis of the siderophore yersiniabactin. Different capsular types are present depending on strain and these are important for survival of the organisms following invasion. The immune response of the host leads to the death of some organisms and endotoxin is released, with resultant clinical signs of pyrexia, weakness, depression and tachycardia. The diseases caused by E. coli in domestic animals are summarized in Table 17.4. Major virulence factors are given in Table 17.5. In this case it is sufficient to isolate E. coli in an almost pure growth from carefully taken samples such as cervical swabs, mastitic milk samples and midstream urine. The culture and presumptive identification methods are shown in Figure 17.15. Pathogenic strains of E. coli are often haemolytic (Fig. 17.18) and as they are strong lactose-fermenters the colonies on MacConkey agar are bright-pink (Fig. 17.19). Eosin methylene blue (EMB) agar is occasionally used in diagnostic laboratories and on this medium E. coli colonies have a unique and characteristic metallic sheen (Fig. 17.20). Other chromogenic agars for the easy identification of E. coli are available commercially. The ‘IMViC’ test (indole+/ MR+/ VP−/ citrate−) is a quick presumptive method of identifying E. coli (Fig. 17.21) as almost no other lactose-positive member of the Enterobacteriaceae gives this combination of results. Biochemical reactions of some clinically significant members of the Enterobacteriaceae, including E. coli, are given in Table 17.6. Molecular virulence typing can be used for isolates such as UPEC which possess defined virulence attributes. Figure 17.19 Escherichia coli on MacConkey agar. Bright pink colonies indicating acid production as a result of the fermentation of lactose. (Neutral red indicator) Figure 17.20 Escherichia coli (right) giving a distinctive metallic sheen on EMB agar distinguishing it from other members of the Enterobacteriaceae such as Salmonella sp. (bottom) and Klebsiella pneumoniae (left). • Fimbrial antigens. Fimbriae are expressed poorly on selective and some types of non-selective laboratory media. E medium (Francis et al. 1982) is advised for F4, F5 and F41. Minca medium (BBL) has been found satisfactory for F6, F5 and F41. Commercial test kits are available for the detection of fimbrial antigens such as a latex agglutination test (Fig. 17.22). Specific antiserum can be obtained for use in a slide agglutination test. Enzyme-linked immunosorbent assays (ELISA) are available for directly measuring the presence of fimbriae-expressing E. coli in faecal extracts. The fluorescent antibody technique, using conjugates prepared against each of the common colonizing-associated antigens, can be used on smears made from scrapings from the ileum of a fresh carcass. Figure 17.22 A latex plate agglutination test for detecting the K99 pili antigens of enterotoxigenic E. coli. Suspensions of three E. coli isolates (tests 1, 2 and 3) and a control antigen have each been placed in a top and a bottom well. Reagent 1 is latex particles coated with monospecific antibody to the K99 antigen and has been placed in all the wells in the top row. Reagent 2 is a suspension of latex particles only and has been placed in all the wells in the bottom row. E. coli (test 1) is positive for the K99 antigen; E. coli (test 2) is negative and E. coli (test 3) has autoagglutinated in the bottom well indicating that the test is invalid for this isolate.
Enterobacteriaceae
Differentiation of the Enterobacteriaceae
Conventional microbiology

+ = positive reaction, − = negative, ‘IMViC’ = indole, methyl red, Voges–Proskauer and citrate tests, TSI = triple sugar iron agar, lysine = lysine decarboxylase test
MacConkey agar
Brilliant green agar
XLD medium
Triple sugar iron (TSI) agar

R = red (alkaline), Y = yellow (acid), H2S+ = hydrogen sulphide produced, H2S− = hydrogen sulphide not produced
Pathogenicity
Genus (species)
Site of isolation and possible pathogenicity
Budvicia aquatica
Water and human faeces
Buttiauxella agrestis
Water
Cedecea species
Rare isolates from human clinical specimens (most commonly from the respiratory tract). Bacteraemia in humans has been reported
Ewingella americana
Rare isolate from human clinical specimens
Erwinia herbicola
This and other Erwinia species are associated with plants as pathogens or saprophytes
Kluyvera species
Water, sewage, soil and milk. Occasionally isolated from human clinical specimens
Yokenella regensburgei (Koserella trabulsii)
Human respiratory tract, wounds, urine and faeces but pathogenicity uncertain
Leclercia (Escherichia) adecarboxylata
Environment, food and water. It has been isolated from human clinical specimens
Lemiorella species
Isolated from human faeces and urine
Moellerella wisconsensis
Human faeces
Obesumbacterium proteus
Found only in contaminated beer. Biogroup 1 is thought to be a brewery-adapted biochemical variant of Hafnia alvei. Unlikely to be pathogenic for animals
Photorhabdus species
Pathogenic for nematodes
Pragia fontium
Isolated from water
Providencia species
Urinary tract of compromised or catheterized human patients, patients suffering from burn infections and diarrhoea. Rarely isolated from faeces of healthy humans
Rahnella aquatilis
Water and from human burn wounds
Tatumella ptyseos
Occasionally isolated from human clinical specimens, mainly from the respiratory tract
Escherichia coli
Pathogenesis and Pathogenicity
Predisposing causes
 The normal flora of the intestines is not fully established
The normal flora of the intestines is not fully established
 They have a naive immune system
They have a naive immune system
 Receptors for the adhesins of enterotoxigenic E. coli are present for the first week of life only in calves and for the first six weeks of life in piglets.
Receptors for the adhesins of enterotoxigenic E. coli are present for the first week of life only in calves and for the first six weeks of life in piglets.
Types of pathogenic E. coli
Enterotoxigenic E. coli
Septicaemic E. coli
Laboratory Diagnosis
Diagnosis of the opportunistic infections caused by E. coli
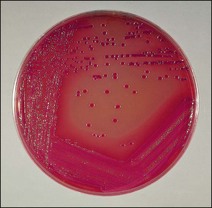

Demonstration of the enterotoxigenic strains
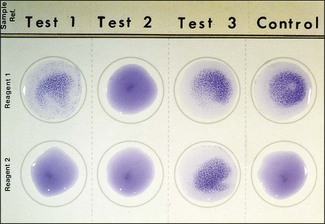
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree