CHAPTER 24 Endocrinology
Endocrine Pancreatic Disorders
Overview
The endocrine pancreas comprises multitudes of islands of cells within the exocrine pancreas known as the islets of Langerhans. The islets represent only 2% of the pancreas and comprise several types of cell. Each cell type secretes a different hormone. The major cell types and the hormones they produce are found in Table 24-1. The close interrelationship among these different cell types allows for direct control of secretion of some hormones by other hormones. For example, insulin inhibits glucagon secretion and somatostatin inhibits the secretion of both insulin and glucagon. Activity of beta cells and production of insulin are of main importance for diabetes mellitus (DM).24
TABLE 24-1 The Major Cell Types of the Endocrine Pancreas and the Hormones They Produce
| Cell Type | Hormone |
|---|---|
| Alpha cells (20%-25% of each islet) | Glucagon |
| Beta cells (60%-80% of each islet) | Insulin |
| Delta cells (10% of each islet) | Somatostatin |
| Gamma cells have two subtypes: | |
| PP (or F) cells | Pancreatic polypeptide |
| D cells | Vasoactive intestinal peptide |
| EE (or enterochromaffin) cells | Serotonin, motilin, substance P |
Diabetes Mellitus
Epidemiology
The prevalence of feline DM is in the order of 1 in 100 to 1 in 200 cases, with higher numbers of cases seen in referral practice than in first opinion practice.5,62,93,95 The number of diabetes cases in cats appears to be increasing (Figure 24-1), and this may relate to higher obesity rates and more cats being fed high-carbohydrate diets.93 Male cats appear to be at greater risk, representing approximately 60% to 70% of all diabetics.5,62,89,93,95 Increasing age also correlates with increasing risk of DM, with approximately 20% to 30% of diabetics diagnosed at 7 to 10 years of age and 55% to 65% of diabetics diagnosed when older than 10 years of age.5,89,93,95 Numerous studies have indicated that Burmese are at higher risk of diabetes than other cats in Australia and New Zealand,5,62,95,114,121 and a United Kingdom survey has indicated likewise.77 This does not appear to be the case in North America, where the Burmese breed appears to be genetically distinct. One North American study indicated an overrepresentation of Siamese cats,89 but a subsequent study did not confirm this.93
Clinical Signs and Diagnosis
According to one authority, “Currently, there are no internationally accepted criteria for the diagnosis of diabetes in cats.”94 Despite this statement, DM is usually recognized as persistent hyperglycemia above the renal threshold for normal cats, which is blood glucose (BG) greater than 16 mmol/L (288 mg/dL), with consistent clinical signs (polyuria, polydipsia, and weight loss). Elevations of BG above the renal threshold result in glycosuria. Caution must be taken to rule out stress hyperglycemia (reportedly as high as 60.4 mmol/L [1087mg/dL]).59 In many cases serum fructosamine concentration will be normal with stress hyperglycemia. Ruling out stress hyperglycemia can also be achieved by treating underlying conditions and then rechecking blood and urine glucose on a subsequent day. This is particularly important if the BG is less than 20 mmol/L (360 mg/dL), intercurrent disease is present that could cause stress hyperglycemia, or typical clinical signs of DM are absent. A second test is not usually required if BG is greater than 20 mmol/L (360 mg/dL).
Evidence of gluconeogenesis (ketosis or ketonuria) supports a diagnosis of DM.94 All diabetic cats in a recent study had at least some elevation of the plasma ketone, beta-hydroxybutyrate. Using a plasma value of 0.22 mmol/L beta-hydroxybutyrate as the cutoff value for diagnosis of DM gave a false positive rate of 9%, whereas 0.58 mmol/L reduced the false positive rate to 1.2%. No cat with moderate or severe stress-related hyperglycemia had beta-hydroxybutyrate concentrations above 0.22 mmol/L.132
Fructosamine is the term used to describe glycated plasma proteins, and serum concentration of fructosamine is related to BG concentration. Serum fructosamine concentration may be used to aid the diagnosis of DM, but care must be taken because in cats a single serum fructosamine concentration measurement most likely reflects the mean BG concentration for approximately the past week (compared with longer durations in other species). Further, serum fructosamine may not exceed the reference range in cats with moderate hyperglycemia of less than 17 mmol/L (306 mg/dL),64 making serial BG testing a more reliable indicator of diabetes. Most cats with newly diagnosed DM will have serum fructosamine levels higher than 400 µmol/L.16
Glycosuria should be considered as part of the diagnostic criteria because this arises as a result of BG above the renal threshold. Urine may be dilute and associated with polydipsia but not necessarily so as some affected cats have concentrated urine. One study showed that 13% of cats with DM had urinary tract infection (UTI),4 and as with other infections, UTI must be treated to aid diabetic control.
Pathophysiology
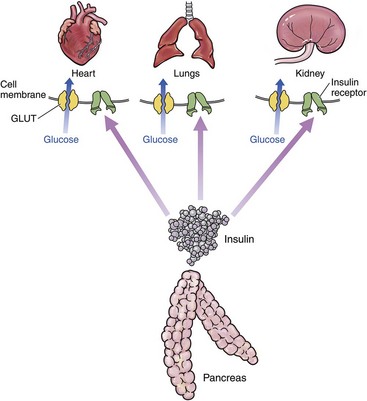
In the healthy individual, for most organs, insulin must bind to insulin receptors at the periphery of cells to allow entry of glucose from the bloodstream into the cell. When insulin binds to the receptor, intracellular mechanisms are activated that result in glucose transporters (contained within intracellular vesicles) moving to the cell membrane. At least 12 glucose transporter proteins (GLUTs) have been described. GLUT4 is responsible for insulin-mediated glucose uptake. GLUT4 vesicles dock on the cell membrane, and then GLUT4 fuses to the cell membrane to allow intracellular diffusion of glucose. This is a complex process mediated by at least three genes in all mammals. Glucose in the bloodstream is mostly from metabolized food.63 These processes have been simplified, as depicted in Figure 24-2. There are two major organs with important differences in glucose metabolism:
1 The liver has enhanced uptake of glucose that is mediated as described previously but also stores glucose in the form of glycogen. In most species hepatic glycogen is split back into glucose (gluconeogenesis) in times of fasting, such as between meals. In cats gluconeogenesis is reported to be active even in the fed state.38 Cats have low levels of glucokinase, an enzyme that facilitates conversion of glucose to glucose 6-phosphate, but high levels of glucose 6-phosphate. Glucose 6-phosphate plays a vital role in glycogen production and glycolysis (energy production).43 The full implications of these feline-specific differences are not yet understood but may prove vital in our evolving understanding of the pathogenesis of feline diabetes.
2 Brain cells are permeable to glucose and can use glucose without the intermediation of insulin. The brain normally uses only glucose for energy and can use other substrates (e.g., fat) for energy only with difficulty (as opposed to other organs). For these reasons it is essential that BG concentration does not fall too low because hypoglycemic shock can result.38
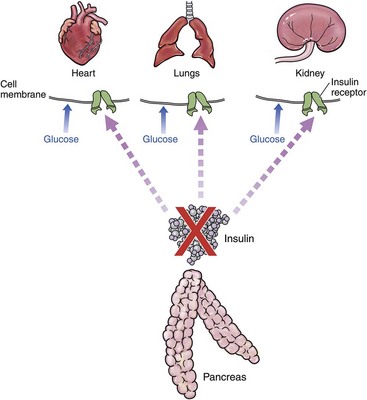
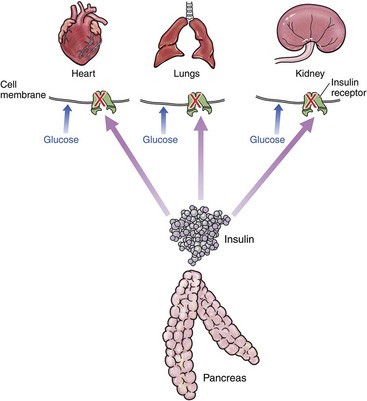
Type 1 DM is due to lack of insulin (Figure 24-3). It is rarely described in cats. It is most often associated with immune-mediated destruction of beta islet cells but has also been described with exocrine pancreatic insufficiency.107,118,131 Type 2 DM is far more common in cats.94 The initiating factor is insulin resistance (Figure 24-4). This may be associated with decreased number of insulin receptors,110 reduced receptor activity,115 direct effect on GLUT4,9 or a combination of factors. Initially, the body responds by producing more insulin. This chronic hyperfunction of the beta islet cells contributes to their eventual failure and the inability to secrete sufficient insulin.
Insufficient insulin production may affect potassium metabolism, insofar as insulin allows potassium to enter cells. In DM the lack of insulin or the lack of receptors means that less potassium is able to get into the cells. Hence there is an increase of potassium in the blood, which is cleared by the kidneys, especially with polyuria. Therefore serum/plasma potassium is even less reflective of the total body potassium than usual. In addition, insufficient insulin production may affect lipoprotein lipase (LPL) activity. LPL works in conjunction with insulin in fatty acid metabolism. The reduced LPL activity may be significant enough to result in lipemia. The lipemia may be due to triglycerides (TGs) or cholesterol, but TGs are more governed by chylomicrons (CMs) and very low-density lipoprotein (VLDL), which have more dependence on LPL activity.127
Underlying Causes
Pancreatic Factors
Even without any overt exocrine pancreatic dysfunction, diabetic cats produce amylin (an amyloid precursor) that is deposited in islet cells, leading to decreased insulin production. In humans genetic assessments have indicated that many of the genes associated with type 2 diabetes are associated with insulin secretion from beta islet cells.102 Additionally, exocrine pancreatic disease occurs as a comorbidity with diabetes, and elevated serum feline pancreatic lipase immunoreactivity (fPLI) has been recognized in diabetic cats.31 Pancreatic diseases such as pancreatitis, pancreatic adenocarcinoma,94 and genetic associations may play a role.61
Peripheral Factors
Epidemiologic studies in cats consistently show DM to be a disease of older cats, with the incidence increasing markedly in cats over the age of 8 years.5,89,93,95 Age associations with insulin resistance are controversial in people with discordant results, most likely because of general health, physical training, and changes in liver size.10,28 Multiple studies have indicated increased insulin resistance in male cats (in particular after neutering) that is consistent with the overrepresentation of males among diabetic cats.3,44,46
There are now several studies indicating that Burmese are more at risk of DM than other cats in Australia, New Zealand, and the United Kingdom.* Breed predispositions are likely to be analogous to the situation for humans, wherein DM is more prevalent in various indigenous groups such as Australian Aborigines, African Americans, and Pima Indians.17,55,67 Genetic studies in humans have identified 20 common genetic variants associated with type 2 diabetes. Most of these genes are associated with regulation of insulin secretion from beta islet cells in response to insulin resistance, but there are also genes related to glucose transport and insulin sensitivity. Many associated genes have unknown roles. In most instances multiple genes are affected in individuals.102 The increasing availability of genome-wide assessments will make searches for specific genes easier in cats, but the complex interactions of multiple genes in humans may make interpretation more difficult in cats, wherein the number of cases assessed will be far less than in studies of people.
Obesity has been directly related to insulin resistance in cats as well as humans3,9 and specifically reduces GLUT4 expression.9 Other consequences of obesity in people, such as decreased insulin signaling and glucose disposal rates,63 are also likely to be relevant in cats. For obesity (and insulin resistance generally) to be associated with type 2 diabetes, the beta islet cells must be unable to compensate fully for the decreased insulin sensitivity.63 Weight loss is therefore an important component of diabetes management.
In comparison to most mammals, cats have very low hepatic activity of the enzyme glucokinase, which plays the important role of acting as a “glucose sensor.” Cats are able to compensate by having elevated levels of glucose 6-phosphate. This altered glucose-sensing pathway in the feline liver may represent an evolutionary adaptation to a low-carbohydrate diet.43 These changes create challenges for all cats to handle the high glucose loads provided by high-carbohydrate diets and, if coupled with insulin resistance (from any cause), can result in diabetes. Recent studies have demonstrated improved remission rates when cats are fed low-carbohydrate/high-protein diets compared with high-fiber diets.7,32,76
Specific infections in people (e.g., hepatitis C) have been correlated with insulin resistance,47 although the reasons for the associations are not elucidated. Additionally, tumor necrosis factor-alpha (TNF-alpha), a cytokine involved in systemic inflammation and the regulation of immune cells, has been demonstrated to play a role in the pathophysiology of insulin resistance.8 Managing underlying infections is an important component of reducing insulin resistance. Azotemia associated with renal disease18 and hyperthyroidism,50 both common diseases in older cats, have also been demonstrated to contribute to insulin resistance. Management of concurrent conditions can therefore aid diabetic control.
Glucocorticoids impair insulin-dependent glucose uptake by peripheral cells and enhance hepatic gluconeogenesis as well as inhibiting insulin secretion from beta islet cells.2A recent study found that high doses of corticosteroids increased serum glucose in all 14 study cats assessed, but clinical signs were seen in only one cat.68
Catecholamines and a number of other hormones released during stress states contribute to the development of hyperglycemia by directly stimulating glucose production and interfering with tissue disposal of glucose. In a normal individual hyperglycemia stimulates the secretion of insulin and inhibits the secretion of glucagon, effects that will diminish the degree of hyperglycemia resulting from direct actions of stress hormones on glucose production and disposal. Cats with impaired islet responses to glucose will be particularly prone to the development of marked hyperglycemia during stress states because they may be unable to respond to the influence of hyperglycemia.39
1 Glucose desensitization: a normal physiologic response that is a rapid and reversible refractoriness of beta cells after short exposure to hyperglycemia
2 Beta cell exhaustion: a reversible depletion of the readily releasable pool of intracellular insulin caused by more prolonged hyperglycemia
3 Glucotoxicity: the slow and progressively irreversible direct toxicity of the beta cells induced by chronic hyperglycemia through functional change and cell death
A continuum exists between beta cell exhaustion and glucotoxicity in that the changes are reversible until a particular point in time.92
Treatment Principles
Recently, a series of 29 specific DM-associated questions centered on both owner and animal were designed as a quality-of-life tool for diabetic cats. The tool was tested with 221 owners of diabetic cats predominantly in the United States and the United Kingdom, about half of whom performed home BG measurements. Nine of the top 10 items judged by owners to have negative impact were related to their own quality of life rather than that of the cat. These were issues such as difficulty boarding the cat, difficulty leaving the cat with family or friends, worry about the disease, worry about hypoglycemia, and changes to work and social life.86
Therefore management of the diabetic cat should be a multipronged approach incorporating insulin, dietary therapy (to reduce carbohydrate load and induce weight loss if the cat is overweight), weaning off corticosteroids when possible, and management of any infection or concurrent condition. If peripheral insulin resistance factors can be overcome, the cat may be weaned from insulin as long as the beta islet cells have not suffered irreversible damage from chronic glucotoxicity. With early intervention and good glycemic control, diabetic remission was achieved in 84% to 100% of cats in two recent studies.73,104 Loss of control of peripheral factors such as a return to a high-carbohydrate diet, recurrence of obesity, or azotemia may result in a return to an insulin-dependent state.
Two recent studies have looked at factors associated with an increased chance of achieving remission. In one questionnaire-based study of owners of diabetic cats treated with glargine insulin participating in an Internet forum, strict glycemic control, administration of corticosteroids before diagnosis, and absence of polyneuropathy were more likely in cats that achieved remission. Factors that were not useful predictors of remission included age, sex, body weight, and presence of chronic renal disease or hyperthyroidism. Cats that achieved remission had a lower mean maximum insulin dose (0.43 U/kg, every 12 hours) than cats that did not achieve remission (0.66 U/kg, every 12 hours).104
In another study of 90 cats with newly diagnosed diabetes, 50% of cats achieved remission after a median time of 48 days. The maintenance insulin was glargine for 47% of cats and protamine zinc insulin (PZI) for 53% of cats. The median duration of remission was 151 days for cats still alive at the end of the study. Insulin was resumed in 29% of cats that had achieved remission, but six of the cats achieving remission did not require insulin again for more than 1000 days. In this study age and cholesterol levels were predictive of remission in multivariate analysis. Increased serum cholesterol decreased the chance of remission by about 65%. For each year of age, the chance of remission increased by approximately 25%. Duration of remission was longer with higher body weight and shorter with higher serum glucose. Cats treated with glargine insulin had an increased chance of remission based on univariate analysis.135
Specifics of Treatment in the “Well” Cat with Diabetes Mellitus
Insulin Therapy
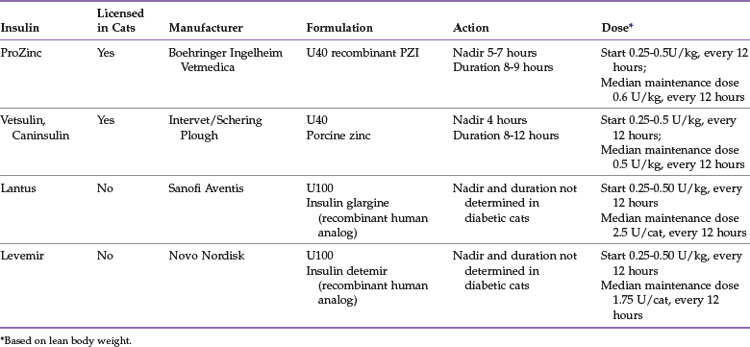
The types of insulins most commonly used in cats can be summarized as follows (Table 24-2):
Rapidly acting, soluble insulin will be discussed below with therapy of diabetic ketoacidosis.
PZI-Vet (IDEXX Pharmaceuticals, Inc.), a 90% beef/10% pork U40 insulin, was commonly used in cats but is no longer commercially available because of the lack of a U.S. Food and Drug Administration–approved source of bovine pancreas. Compounded PZI cannot be recommended because the potency varies from batch to batch, making long-term regulation difficult. A replacement product based on recombinant human PZI insulin was developed and approved for cats as ProZinc (Boehringer Ingelheim Vetmedica) in 2009. One study that compared recombinant PZI (PZI-R, now sold as ProZinc) to PZI-Vet was conducted in six private feline specialty clinics. A total of 50 cats with DM and stable glycemic control on PZI-Vet (for at least 90 days) were switched to PZI-R for 30 days at the same dose rate and interval. In the 47 cats completing the study, there were no significant differences in body weight or serum fructosamine concentrations at days 15 or 30 compared with day 0. The researchers concluded that PZI-R provides glycemic control that is comparable to that of PZI-Vet when used at the same dose and dosing interval.87
A prospective, uncontrolled 45-day clinical trial evaluating the efficacy of PZI-R (ProZinc) for controlling glycemia in cats with newly diagnosed, untreated diabetes (n = 120) and cats with previously treated, poorly controlled diabetes (n = 13) was also published recently. Treatment was started at 1 to 3 U/cat every 12 hours (0.22 to 0.66 U/kg every 12 hours), and the dose was adjusted at re-evaluations on the basis of the history and results of physical examination, body weight, and BG curves. Feeding the same diet to all cats was not attempted, although most cats were fed a high-protein/low-carbohydrate diet. The mean time of BG nadir was between 5 and 7 hours, and subsequent BG concentrations were rising in most cats by 9 hours after administration. By day 45 the owner’s subjective assessment of polyuria and polydipsia had improved in 79% of cats; 89% of cats had good body weight; and 9-hour mean BG concentration, serum fructosamine concentration, or both had improved in 84% of the cats compared with day 0. Biochemical hypoglycemia (BG less than 4.4 mmol/L [80 mg/dL]) occurred at least once in 64% of cats, and clinical signs of hypoglycemia were confirmed in two cats.83
Vetsulin/Caninsulin, a porcine lente insulin, is a mixed insulin zinc suspension containing 30% amorphous zinc insulin (which is rapidly absorbed and has a short duration of activity) and 70% crystalline zinc insulin (which is absorbed more slowly and has a longer duration of activity). The onset and duration of action are shorter than that of PZI in cats, with a BG nadir at about 4 hours after injection, and a duration of about 8 to 12 hours.72,74
A 12-month prospective study of Vetsulin/Caninsulin was conducted with 25 cats, most of which were newly diagnosed (n = 15), whereas the remainder were poorly controlled on other therapies. Cats with BG over 19 mmol/L (over 342 mg/dL) were started at 0.5 U/kg every 12 hours and cats with BG less than 19 mmol/L (less than 342 mg/dL) were started at 0.25 U/kg every 12 hours. No specific diet was fed. After an initial 6-day examination period, the cats were re-examined at 4, 8, 12, 26, and 52 weeks. Increases in insulin dose were made as needed, with a target BG nadir of 5 to 9 mmol/L (90 to 162 mg/dL). The median insulin dose was 0.5 U/kg every 12 hours (range 0.1 to 1.9 U/kg every 12 hours), and only two cats required doses higher than 1 U/kg every 12 hours. During the study period seven cats went into remission within 15 weeks, and none relapsed during the 12 months. Of the 18 cats that did not go into remission, 13 reached the water intake target established for ideal diabetic control (less than 20 mL/kg per day for canned diets, less than 70 mL/kg per day for dry diets). The control of clinical signs in the cats that did not achieve remission was deemed either excellent or good. It took approximately 3 months for significant resolution of clinical signs.75
Another recent prospective, multicenter, nonblinded, open study followed 46 cats with diabetes (either newly diagnosed [n = 39], or previously treated but poorly controlled [n = 7]) during treatment with Vetsulin/Caninsulin. The cats were monitored for about 16 weeks (stabilization phase), with additional monitoring of some cats (n = 23) for a variable period. The starting dose for each cat was based on the initial BG concentration: 0.25 U/kg if the BG was less than 20 mmol/L (360 mg/dL), and 0.5 U/kg if greater than 20 mmol/L (360 mg/dL). The maximum starting dose did not exceed 2 U/dose, and dose rates greater than 0.5 U/kg twice daily were not recommended during the first 3 weeks of treatment. No specific diet was used for all cats. At the end of the stabilization phase, 15% of cats achieved clinical remission. None of these cats had been treated previously for diabetes. Approximately 60% of the remaining cats were clinically stable after 3 to 4 months of treatment, a finding in line with studies published previously using a variety of insulins. Clinical signs of hypoglycemia were observed in nine cats during the stabilization period and were significantly associated with a dose of 3 U/cat or over 0.5 U/kg administered every 12 hours.79
PZI and lente insulins, when administered twice daily, resulted in marked hyperglycemia (>18 mmol/L, 324 mg/dL) for several hours before each insulin injection in one study using nine healthy cats.72 The continued (though intermittent) hyperglycemia of such therapy most likely contributes to continued glucotoxic effects of permanently damaging beta islet cells and would explain why higher remission rates have been seen with glargine73,104 and detemir.106
The recommended starting dose of glargine is 0.25 U/kg every 12 hours for cats with BG less than 20 mmol/L (less than 360 mg/dL), and 0.50 U/kg every 12 hours when the BG is over 20 mmol/L (over 360 mg/dL). One study in nine healthy cats showed that the nadir with glargine occurs approximately 12 to 16 hours after injection,72 whereas another study in five healthy cats using a euglycemic clamp showed the peak effect occurred between 2 and 9.75 hours.33 Although it may appear that there is a discrepancy in reported time to peak action for glargine, it is likely a result of the very different study designs and different parameters each study used to define time to peak insulin action (nadir versus maximal glucose infusion rate). Put simply, the first study reported the time that nadir glucose occurred (based on actual BG concentration), whereas the second study reported the time of peak glucose infusion rate (to maintain normoglycemia). Further, these studies were performed with small numbers of healthy cats, and whether a similar response would be seen in diabetic cats is unknown. Individual cats with a glargine nadir after 12 hours may achieve adequate glycemic control with once-daily dosing, but twice-daily dosing is associated with better glycemic control and higher remission rates.73,124 Hence twice-daily dosing is recommended for most cats.
Detemir is an insulin analog that binds to albumin and is released slowly for a long duration of action. On the basis of studies by the manufacturer in humans, variations in BG with detemir may be even less pronounced than with glargine. The duration of action and peak effect of detemir and glargine were evaluated in a study of five healthy cats. Definitive conclusions are hard to reach with small numbers of healthy cats, but the authors assessed that although glargine has a more rapid peak effect than detemir, it was more variable from cat to cat than detemir (glargine: 120 to 585 minutes; detemir: 370 to 575 minutes). The duration of action was longer for detemir in three cats but longer for glargine in two cats.33
It is important not to mix or dilute detemir or glargine insulin because the duration of action depends on the pH of the product. Glargine (Lantus) is available in 10-mL vials and a 3-mL SoloSTAR pen that measures in 1-unit increments. However, the pen is expensive, cannot be refrigerated, and expires in 1 month. The cartridges for the pen can be dispensed to be used as individual 3-mL vials. If refrigerated, open vials of glargine or detemir can be used for approximately 6 months.96 Detemir is available in 10-mL vials and a 3-mL FlexPen that measures in 1-unit increments. Once in use, the FlexPen is stored at room temperature and expires in 42 days. These insulins should be clear and colorless; if cloudiness, discoloration, or clumping is noted, the product should be discarded. It is not necessary to shake or rotate the vial before use. Glargine and detemir are not licensed for use in veterinary patients.
Two studies with the same protocols, except varying insulin type, found similar remission rates for glargine (84%)104 and detemir (81%)106 in diabetic cats starting therapy within 6 months of diagnosis. Remission rates were much lower if insulin therapy was started more than 6 months after diagnosis. The insulins were administered twice daily, and a low-carbohydrate diet was fed. Median time to remission was 1.7 months for cats receiving detemir and 1.9 months for cats receiving glargine. Most of the cats achieving remission were able to stay off insulin, and the median duration of remission was 10.8 months for glargine and 1 year for detemir. Although biochemical hypoglycemia was common, clinical hypoglycemia was rare, with only a single event with mild signs in one cat on each insulin. The median detemir dose was 1.75 U/cat every 12 hours, and the median glargine dose was 2.5 U/cat every 12 hours.
A direct comparison of insulin therapies in 24 newly diagnosed diabetic cats found remission rates were higher in those cats receiving glargine (100%) than PZI (38%) and lente insulin (25%) during the 16-week study period.73 The initial dose of insulin was 0.5 U/kg ideal body weight if the BG on admission was greater than or equal to 360 mg/dL (20 mmol/L), and 0.25 U/kg if BG was less than 20 mmol/L (360 mg/dL). BG was measured every 2 hours for 12 hours for each cat for the first 3 days of treatment to ensure that cats did not become hypoglycemic. No increase in insulin dose was made during the first 3 days, even if persistent hyperglycemia was present. During the study two cats treated with lente insulin and one cat treated with PZI had severe clinical hypoglycemia requiring intravenous glucose therapy. None of the glargine-treated cats exhibited signs of hypoglycemia, although many had biochemical hypoglycemia (BG less than 3 mmol/L [less than 54 mg/dL]) without clinical signs. By 16 weeks all eight cats in the glargine group had achieved remission, whereas two of eight cats in the porcine lente group and three of eight cats in the PZI group had achieved remission. The probability of remission in newly diagnosed patients fed a low-carbohydrate/high-protein diet was significantly greater for cats treated with glargine than cats treated with PZI or lente in this study.
One 12-week study of 13 diabetic cats found no significant difference in remission rates in cats receiving lente insulin (three of seven, 43%) compared to glargine (one of six, 17%). However, approximately half the cats enrolled in this study were not newly diagnosed with diabetes, cats received glargine only once daily but lente insulin twice daily, and monitoring was only every 4 weeks (after the initial 4 weeks).124
Insulin Dosing and Monitoring Protocols
Managing diabetic cats with insulin with the objective of diabetic remission is a balancing act requiring sufficient insulin for glycemic control without causing hypoglycemia. The recommended starting dose for most insulins is 0.25 U/kg to 0.5 U/kg, with higher doses preferred for those cats with higher BG (more than 20 mmol/L [360mg/dL]) recognized at diagnosis.71,104,106 Frequent re-evaluations are required during the initial stabilization period, as outlined later and in Box 24-1. Typically, most cats first go through a phase where insulin dose is increasing, then stabilize when insulin dose is consistent, and then for cats achieving remission, a phase of decreasing insulin dose.
BOX 24-1 Suggested Treatment and Management Protocol for Cats with Nonketotic Diabetes Mellitus
• Evaluate for concurrent diseases with minimum database (complete blood count, serum biochemistry profile, urinalysis, urine culture, total T4, feline pancreatic lipase immunoreactivity).
• Hospitalize for 1 day to begin insulin therapy; measure blood glucose every 3-4 hours, monitor for hypoglycemia.
• Consultation with owner to demonstrate insulin handling and injection techniques, discharge with written instructions on diet, monitoring for hypoglycemia, monitoring appetite and water intake, and so on. Introduce the concept of home blood glucose monitoring.
Three days later (this step not necessary for Lente insulin).
• Hospitalize for 1 day; measure blood glucose every 3-4 hours, and monitor for hypoglycemia.
• Consult with owner to confirm insulin handling and injection techniques.
• Re-evaluate body weight and condition, and perform full physical examination.
• Hospitalize to perform blood glucose curve, and adjust insulin dose as needed; preferably use the same portable blood glucose meter that the owners would eventually use at home, and validate by comparison to in-hospital chemistry analyzer.
• Consult with owner on home management of diet, and identify and correct any problems with insulin administration.
Repeat weekly in hospital blood glucose curves.
• Until owner feels confident to perform at home (can show owner each week)
• Or until appropriate nadir is achieved
• Re-evaluate body weight and condition, and perform full physical examination.
• Hospitalize to perform blood glucose curve, and adjust insulin dose as needed.
• Consult with owner on any problems encountered with home care or monitoring.
• Discuss home blood glucose monitoring; demonstrate techniques, and provide supplies and written instructions.
• Owner performs 12-hour blood glucose curve weekly until stabilized and telephones hospital to provide results.
Three months later and every 3 months subsequently
• Re-evaluate body weight and condition, and perform full physical examination.
• Evaluate home monitoring, including blood glucose measurements.
• Hospitalize for blood glucose curve.
• Compare hospital blood glucose curve and home blood glucose curve.
• Make insulin dose adjustments as required.
• Make adjustments to dietary therapy as required.
• Owner performs home blood glucose curve once monthly.
It is the authors’ experience that hypoglycemia is rare initially, and if it is recognized biochemically within the first few days of therapy, there is a very low probability of clinical signs. Starting most diabetic cats at 0.5 U/kg increases the chance of establishing timely glycemic control. However, starting a cat on this dose of insulin requires close monitoring from day 1. When glargine was first introduced to management of feline diabetes, initial protocols called for BG testing every 4 hours for each of the first 3 days and then weekly.71 It is usually more practical to perform BG curves on day 1 and day 3. Long-acting insulins are marketed in human medicine as being peakless; this is not the case (at the very least in cats),72 and “spot” checks of BG are not adequate or appropriate to maintain glycemic control of diabetic cats. Serial BG measurements (BG curves) are the most effective monitoring technique to establish what is happening with the cat’s glucose homeostasis. Fructosamine assays are a crude measure reflecting the mean BG concentration for approximately the past week (compared with longer time periods in other species)64 and are not an effective measure for tight management of glycemic control. High serum fructosamine concentrations indicate poor glycemic control but give no information about the nadir BG concentration and cannot be used to determine whether the insulin dose must be increased or decreased. In addition, some disease states, such as hyperthyroidism, affect fructosamine concentrations. Cats with overt, uncontrolled hyperthyroidism may have fructosamine concentrations below the normal range, probably as a result of metabolic changes.101
There is no uniformly recognized BG monitoring protocol for diabetic cats. High remission rates have been achieved with weekly in-hospital monitoring of BG curves (stretching to every 2 weeks after 4 weeks)73 as well as with home monitoring up to daily and insulin dose changes instituted as often as every 3 days.104,106 The following schedule is suggested for BG monitoring either at home or in the hospital (serial measurements are taken on each day):
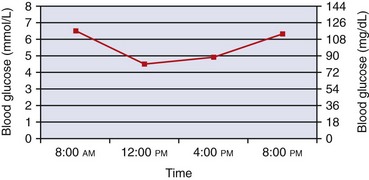
BG can be evaluated every 3 to 4 hours. After an owner obtains a BG curve at home, especially during the stabilization period, the results should be reported to the clinician or veterinary nurse/technician. An appropriate BG curve for a stable diabetic cat being treating with long-acting insulin (e.g., glargine or detemir) is shown in Figure 24-5. A cat with a curve similar to the one illustrated needs no dose adjustment. However, cats can have such a curve yet need a dosage reduction (or increase) at a subsequent evaluation. In the early stages of treatment with long-acting insulins, the BG curve may resemble a curve obtained with lente insulin (Figure 24-6) with more distinct peaks but will usually flatten within 2 to 3 weeks. The slow absorption of glargine and detemir can result in atypical shaped curves with elevations of BG in the middle of the day (but this usually only occurs when there is minimal change in BG). Any dose adjustment decisions should be based on the lowest BG level of the day and should always take into account the patient’s clinical assessment (e.g., body weight, body condition score, appetite, water intake, and urine output).
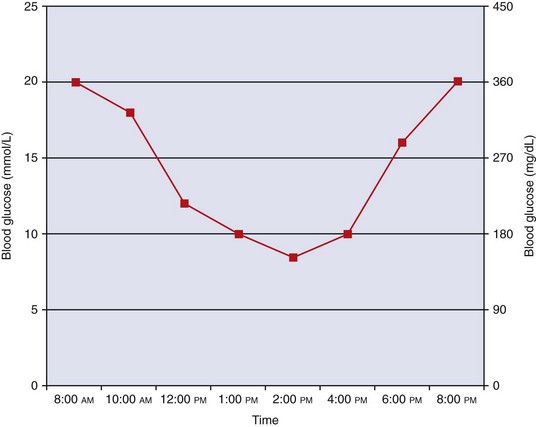
An appropriate curve for a stable diabetic cat being treating with an intermediate-acting insulin (e.g., Vetsulin/Caninsulin or Humulin-N) is shown in Figure 24-6. Note the extended periods of time that BG remains above 15 mmol/L (above 260 mg/dL).
Common sense should be used when making any insulin dose adjustments. Suggested guidelines for dosage adjustments are shown in Table 24-3. Some guidelines for long-acting insulin dose adjustments also include recommendations based on the pre-insulin BG. The reason for monitoring is to assess the effect that particular dose of insulin has had, so changing the dose on the morning of testing defeats that purpose. The exception to this rule is when BG concentration is low before administration of insulin, in which case that dose should be skipped and the dose administered 12 hours later should be lower. As for long-acting insulins, dose adjustments should always take into account the patient’s clinical assessment (e.g., body weight, body condition score, appetite, water intake, and urine output).
TABLE 24-3 Suggested Guidelines for Insulin Dose Adjustment Based on Blood Glucose Curve Results
| Insulin Type | Glucose Concentration | Insulin Dose Recommendation |
|---|---|---|
| Vetsulin Caninsulin | Nadir <3 mmol/L (54 mg/dL) | Reduce by 50% |
| Nadir 3-4.5 mmol/L (54-81 mg/dL) | Reduce by 1 U | |
| Nadir 4.5-7 mmol/L (81-126 mg/dL) | Reduce by 0.5 U | |
| Nadir 7-10 mmol/L (126-180 mg/dL) | No change | |
| Nadir 10-12 mmol/L (180-216 mg/dL) | Increase by 0.5 U | |
| Nadir >13 mmol/L (234 mg/dL) | Increase by 1 U | |
| Pre-insulin <12mmol/L (216 mg/dL) | Withhold and check for remission | |
| Lantus Levemir ProZinc | Nadir 4.5-9 mmol/L (81-162 mg/dL) | No change |
| Nadir <4.5 mmol/L (81 mg/dL) | Reduce by 0.5 U | |
| Clinical hypoglycemia | Reduce by 50% | |
| Pre-insulin <4.5mmol/L (81 mg/dL) | Withhold and check for remission |
Adapted from Rand J, Marshall R: Feline diabetes mellitus: which insulin do I choose & how do I adjust the dose? Proc ACVIM Forum, 2006; Nelson RW, Henley K, Cole C: Field safety and efficacy of protamine zinc recombinant human insulin for treatment of diabetes mellitus in cats, J Vet Intern Med 23:787, 2009; Marshall RD, Rand JS, Morton JM: Treatment of newly diagnosed diabetic cats with glargine insulin improves glycaemic control and results in higher probability of remission than protamine zinc and lente insulins, J Feline Med Surg 11:683, 2009.
Changing Insulins
• From compounded PZI to ProZinc: Reduce the dose slightly, and perform a BG curve after 1 week and re-evaluate every 1 to 2 weeks until stable.
• From PZI-Vet to ProZinc: No dose adjustment is required; perform a BG curve 1 week later.
• From Vetsulin to ProZinc, Lantus, or Levemir or from PZI to Lantus or Levemir: It is not possible to extrapolate a dose, so the patient must be regulated on the new insulin as for a newly diagnosed patient; it is appropriate to start with 0.5 U/kg every 12 hours for most insulins.
Glucometer Choice
Handheld glucometers to assess BG at the point of care have become standard in the management of DM in animals as well as humans. Only a limited number of glucometers have been critically assessed in the veterinary literature, with varying results.15,22,65,125,136 Accuracy is assessed by precision (variation of the individual glucometer for a particular sample) and bias (variation from a reference laboratory measurement). Accuracy is most often poor when very low or very high BG concentrations are measured.
Most glucometers are calibrated for human use. The distribution of glucose between plasma and red blood cells is different in feline blood compared with human blood. Glucometers for human use generally read lower (approximately 1 to 2 mmol/L [18 to 36 mg/dL]) than measurements from an automated chemistry analyzer. To further confuse matters, whole BG (measured with a glucometer) is approximately 10% lower than glycolysis-inhibited plasma glucose (“gray-top” tube samples); also compared with whole BG measurements, serum glucose reduces by approximately 5% to 7% per hour as a result of continued metabolism of glucose by the red blood cells.97 Even glycolysis inhibitors contained in gray-top blood tubes (e.g., sodium fluoride/potassium oxalate) result in reduced plasma glucose readings when compared with promptly spun serum samples.122 Additionally, capillary BG measurements can be between 10% and 24% higher than venous whole BG in a nonfasted state.58 Despite these multiple variations, studies in cats have shown minimal variation between samples obtained from the pinna versus peripheral vein,117,126 and a further study assessed glucometers by directly comparing capillary pinna samples to venipuncture collected samples.136
The highest degree of accuracy among the glucometers tested to date has been shown with the AlphaTRAK glucometer (Abbott Laboratories), one of the few machines calibrated for use in veterinary patients. Although the AlphaTRAK is not as easy to use as human-medicine glucometers, it requires a very small sample volume (0.3 µL) and the test time is only 15 seconds. However, care must be taken with this glucometer insofar as it may be calibrated for canine or feline blood and accuracy is greatly diminished if it is set incorrectly.136 The Ascencia Elite (Bayer Healthcare) glucometer has also been demonstrated to be accurate125,136 but reads lower than the AlphaTRAK and reference laboratory samples,136 which potentially means that lower nadirs will be recognized.104,106 Other veterinary glucometers include GlucoPet and GlucoVet (Animal Diabetes Management, Janesville, Wisconsin), although they have not been critically assessed as of this writing. Glucometers should be selected carefully for use in practice and owners’ use at home, with the clinician and owner having an awareness of the bias of the glucometer compared with laboratory measurements of glucose. Ideally, the clinician would calibrate each glucometer used at home against the hospital’s own blood chemistry analyzer or calibrated glucometer.
Home Monitoring
Perceived benefits of home BG curves include obtaining samples in a less stressful environment where the cat’s food intake should be normal and the ability to perform more frequent BG monitoring. Many owners have the dedication and the ability to perform BG curves at home, but many do not realize this at first and can be intimidated by this prospect. As already noted, success is most likely when additional tasks for the owner of a newly diagnosed diabetic cat are sequentially introduced. Showing owners how to take a BG sample at the same time they are shown how to give an insulin injection for the first time is likely to reduce compliance. Some owners are happy to proceed with sampling their cat’s BG after being shown only once; others need reassurance and multiple demonstrations. Common reasons for their initial reluctance to attempt home BG monitoring include fear of hurting the cat, fear of taking a blood sample, and concern over cost and time commitment. Watching an owner perform the procedure allows the clinician to identify and correct problems. Providing reliable and easy to understand educational resources as well as ready access to telephone support is also important.119 Long-term compliance with home BG monitoring is reportedly good, with 65% of owners performing it regularly in one study. Most owners in that study performed home BG curves every 2 to 4 weeks. Home monitoring provides owners with more confidence in their ability to manage their cat’s disease.54
In one study 12% of owners made changes to their cat’s insulin dose without consulting the clinician. Owners should be cautioned against the temptation to make frequent changes to insulin dose and should make changes only after consultation with a veterinarian.54 Another study found that approximately one fourth of cats had higher BG concentrations for 2 to 3 days after a glargine insulin dose increase.104 The reasons for this are not known but may be associated with a lag time before feedback mechanisms adjust glucose homeostasis. The authors’ experience is that the instinct of most owners is to further increase the insulin in this circumstance, sometimes on three consecutive days, which leads to an increased risk of hypoglycemia.
It must be emphasized that management of the diabetic cat is a team effort that includes the owner, the clinician, and often support staff such as veterinary technicians and nurses. Regular re-evaluations should be established to monitor weight and body condition score, look for underlying issues (e.g., dental infection), and confirm BG findings and discuss the owner’s findings at home (see Box 24-1). One study has shown that the number of re-evaluations did not differ significantly between cats managed with and without home monitoring.54 Once glycemic control has been achieved, diabetic cats should be re-evaluated approximately every 3 months.
BG concentrations measured at home have been compared with those measured in hospital in one study; surprisingly, hospital measurements were lower than those obtained at home.13 This may have been due to inappetence in hospital or stress from the less experienced owner taking the sample at home. In about 40% of cats, the hypothetical treatment decisions based on the two methods of generating BG curves did not agree. Alternatively, it has been demonstrated that BG readings can be significantly higher in hospital (Figure 24-7).100
Another study compared paired BG curves generated at home on 2 consecutive days in seven diabetic cats. In the second part of the study, two BG curves generated at home and one BG curve generated in hospital were compared. The results demonstrated considerable day-to-day variation in BG curves, even those obtained at home. The day-to-day variability between the home and hospital BG curves was not larger than between the paired home BG curves. There was less variability in the home BG curves obtained from cats with good glycemic control than from cats with moderate to poor glycemic control. These findings would indicate that a single BG curve may not always be reliable for making treatment decisions, whether performed in the hospital or at home.1
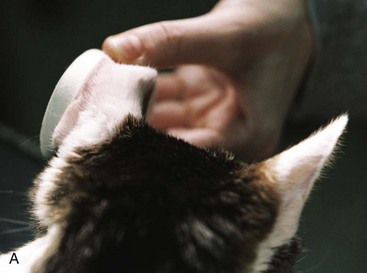
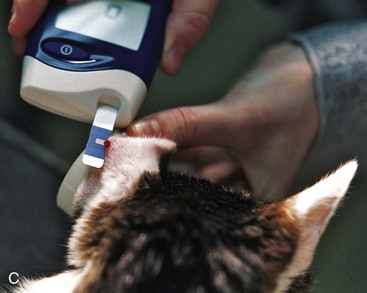
Sites that may be used for home BG sampling are the ear54,100,126 and the foot pads.134 The pinnae (either the haired or nonhaired surface) are most commonly used to obtain samples (Figure 24-8). The ear should be warmed by rubbing or holding a heated cotton ball against the ear (the cotton ball can be heated by placing in a standard microwave for 10 seconds). The ear should not be cleaned or disinfected with alcohol. In longhaired cats, it may be helpful to shave a small portion of the pinna to improve visibility or to apply a thin film of petroleum jelly (petrolatum) to prevent the blood from dissipating into the fur. The opposite side of the ear can be stabilized with a cylindrical object, such as a roll of bandage tape. Then a lancet device or a 25-g hypodermic needle (without a syringe attached) can be used just near (but not directly over) the marginal ear vein to produce a blood drop. The test strip of the glucometer is then touched to the drop to absorb blood. While the machine is processing the sample, a piece of gauze or cotton ball can be used to apply pressure to the site to stop bleeding.
Lancet devices are readily available from most pharmacists, and it is appropriate for the clinicians to have several brands in stock to try on individual cats because cats react differently. Two methods of capillary sampling from the ear have been described.126 One method, using a conventional lancing device, was evaluated only in dogs but has also been used in cats. Alternatively, a lancing device that uses negative pressure (Ascensia Microlet Vaculance, Bayer Diagnostics) to help ensure a blood drop of adequate size can be used.99
As may be expected, certain difficulties are commonly encountered when owners initially begin home BG monitoring, such as the need for assistance to restrain the cat, numerous attempts to produce a blood drop of sufficient size, and resistance from the cat. These difficulties tend to decrease over time as the owner gains experience with the technique.119
Continuous glucose monitoring systems have been designed to help human diabetics improve glycemic control and have recently been introduced in veterinary medicine. These devices measure interstitial glucose concentrations through a subcutaneously placed sensor. The sensor and transmitter are secured to the patient’s back or side by bandaging. The data are relayed to a monitor that must be placed within several feet of the patient to record readings. Glucose readings are taken every 5 minutes, allowing for a large number of readings in a 24-hour period. Validation and use of these devices in cats have been described for both in-hospital and at-home use. Currently, drawbacks include the need for calibration every 12 hours and the need to remove the sensor after 72 hours, as well as the cost.*
Glucotest granules (Purina Veterinary Diagnostics) are a litter additive designed to detect glucosuria through a color change. In one study of the Glucotest granules, the glucose concentration was measured accurately in 29 of 48 feline urine samples. Of the inaccurate measurements, most were overestimates in the 2.8 to 16.6 mmol/L (50 to 300 mg/dL) range. Measurements taken at 8 hours were more accurate than immediate measurements. However, the researchers used frozen urine with added glucose rather than urine from diabetic cats, which may affect results.30
Urine dipsticks are commonly used to measure glucose as well as other urine chemistries and may be used by owners at home. The accuracy of these sticks for feline urine is not well investigated. The ability of one brand (Bayer Multistix) to detect glucosuria in feline urine was determined by comparison to a chemistry analyzer. Sensitivity (73%), specificity (97%), and positive (73%) and negative (97%) predictive values were calculated. Overall, the accuracy of the test strip for classifying the urine glucose concentration in the correct interval was 91% (compared with 59% in dogs). Inaccuracies tended to be underestimates. The study was performed on urine samples submitted to a laboratory, however, whereas most owners are testing urine voided into a litter box, which may affect results.6 Well-regulated diabetic cats receiving PZI or lente insulin should have urine glucose readings between trace and 1+, although well-regulated cats on glargine or detemir insulin may have negative or trace readings. Persistent values outside this range should prompt the owner to seek veterinary advice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree