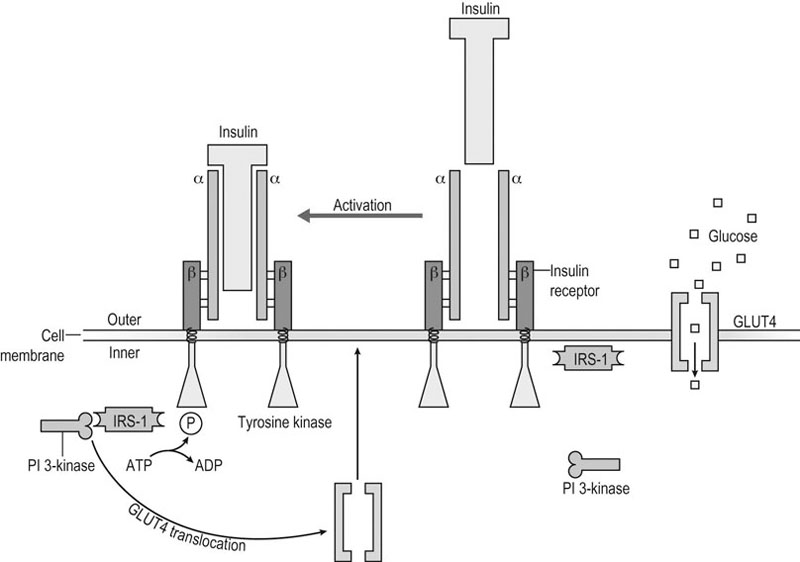
Exercise is a stress situation that challenges homeostasis for which the body must find a new dynamic equilibrium. This dynamic process requires, among other things, adaptive responses of the hormonal systems.1 For instance, the neuroendocrine reflex arch is characterized mainly by the activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, whereas the autonomic branch consists of the activation of the hypothalamic–sympathetic–adrenomedullary axis. Interestingly, both the neuroendocrine and the autonomic efferent arcs of the generalized stress response converge on the adrenal glands, albeit on different timescales and with different final endpoints: the autonomic response occurs rapidly, triggered by sympathetic neural outputs that stimulate the secretion of adrenomedullary catecholamines (like adrenaline), and the neuroendocrine response occurs on a slower timescale, triggered by hypothalamic–pituitary hormone output and resulting in adrenocortical glucocorticoid secretion.2,3 This response, in turn, stimulates other endocrine glands to secrete hormones that potentiate fuel mobilization and regulate water and electrolyte concentrations. As duration of exercise increases, nutrient and ion (electrolyte) concentrations also influence hormonal responses.4 The study of exercise-associated hormonal alterations is of growing interest because of the implications for adaptation, performance, welfare and health. Interpretation of results, however, is difficult because of the numerous factors that affect plasma hormone concentrations. Changes in plasma volume and/or hormone clearance rate, as well as the influence of timing and method of blood sampling and analysis must be considered when evaluating endocrine responses to exercise. Other factors that must be considered are the design of exercise protocols, and various characteristics of the horses studied.4 Physical activity plays an important role in tissue anabolism, growth and development, but the mechanisms that link patterns of exercise with tissue anabolism are not completely understood. One of the unique features of exercise is that it leads to a simultaneous increase of antagonistic mediators. For instance, on the one hand, exercise stimulates anabolic components of the growth hormone (GH)-insulin-like growth factor-I (IGF-I) axis, but, on the other hand, exercise is also associated with an increase in catabolic pro-inflammatory cytokines. This emphasizes the importance of optimal adaptation to exercise. The very fine balance between the anabolic and inflammatory/catabolic response to exercise will determine the effectiveness of exercise training and the health consequences of exercise.5 The effectiveness of physical training depends on the training load and on the individual ability to tolerate it, and an imbalance between the overall strain experienced during exercise and the athlete’s tolerance of such effort may induce overreaching/overtraining syndrome5,6 or undertraining.5 In addition, the stress of exercise might inhibit the gonadal function especially concerning the female human athlete that many authors describe as the so-called ‘exercise-related female reproductive dysfunction’.1 In comparison, moderate exercise (six days/week) in mares suppressed peak LH concentrations, decreased the number of follicles sized 6–20 mm, and lengthened the interovulatory interval,7 whereas repeated strenuous exercise in stallions induced negative influences on semen quality.8 Phylogenetically, GH is an ancestral hormone that has been identified in the pituitary of primitive vertebrates.9 In addition, signal transducers and activators of transcription (STAT) 5, a principal intracellular mediator of GH signaling, exhibits a very high degree of homology between invertebrate and prevertebrate species, reflecting the ancient nature of the GH/STAT signaling system.10 Intriguingly, it also appears that insulin-like peptides, such as IGF-I and proinsulin, are much older than both the pancreas and insulin.11 In line with this phylogenetic hierarchy, GH, together with prolactin and human placental lactogen, stimulates β-cell proliferation, insulin gene expression, and insulin biosynthesis and secretion.12 GH may be viewed as the primary anabolic hormone during stress and fasting. During fasting, GH contributes to the switch from carbohydrate utilization to lipolysis and lipid oxidation.13 GH exerts anabolic effects directly as well as indirectly by stimulation of hepatic IGF-I production. When subjects are well nourished, the GH-induced stimulation of IGF-I and insulin are important for anabolic processes. The most prominent metabolic effect of GH is a marked increase in lipolysis and concurrent FFA concentrations. GH is a counter-regulatory hormone that antagonizes the hepatic and peripheral effects of insulin on glucose metabolism via mechanisms involving the concomitant increase in FFA flux and uptake. The ability of GH to induce insulin resistance (IR) is significant for the defense against hypoglycemia, for the development of ‘stress’ diabetes during fasting and inflammatory illness, and perhaps for the ‘dawn’ phenomenon (the increase in insulin requirements in the early morning hours).13 GH is a protein hormone secreted by the anterior pituitary gland in a pulsatile fashion under the regulation of two hypothalamic peptides: GH-releasing hormone (GHRH) stimulates GH synthesis and secretion while somatostatin inhibits GH release. Whereas GHRH is required for the initiation of GH pulses, the amplitude of GH pulses is modulated by somatostatin, resulting in a pattern of volleys of GH-secretory pulses with intervening periods of relative secretory quiescence. The amplitude and frequency of GH-secretory pulses are regulated by a complex array of external and internal stimuli14,15 with exercise and sleep being robust physiological stimulators of GH secretion.16,17 As a protein hormone, GH is rather species specific, is not lipophylic, and binds to a specific cell membrane receptor. In the normal human population, a gradual and progressive fall in spontaneous GH (and associated IGF-I) secretion occurs with increasing age; this age-associated decrease in GH secretion is termed ‘the somatopause’. It remains to be determined whether this fall in GH secretion is an important physiological safety event of the normal aging process by preventing relative GH excess, or whether it marks the development of GH deficiency which would benefit from GH replacement.16 In comparison, chronic recombinant equine GH administration (up to 12.5 mg/d SC for 43 days) did not affect body weight or muscle mass dimensions in geriatric horses.18 The development of computerized algorithms to quantify episodic GH release has offered new insights into the pathophysiological regulation of GH secretion in humans19,20 and horses.20b So-called pulse detection algorithms and deconvolution analysis were developed to aid in the unraveling of GH release dynamics. A pulse detection program19 was designed to describe concentration peaks in a data series. However, it does not provide information on the two events that together determine the concentration peak: namely, hormonal secretion and clearance. Deconvolution analysis will render a quantitative description of GH secretory and clearance dynamics for the number, amplitude, duration, and temporal locations of all significant underlying secretory bursts and the half-life of endogenous GH disappearance.21 In order to use deconvolution analysis with sufficient accuracy, it is of importance to fulfill the following criteria necessary to obtain a valid GH concentration curve:22 1. a sensitive GH assay with a known precision of low, middle, and high hormone concentrations 2. a long enough sampling period during which pulses can be detected 3. optimally, the sample frequency should be approximately 0.25 times the hormone half-life. In order to meet the last criterion, the disappearance half-life of the hormone should be known.21 Mean endogenous and exogenous GH half-life in yearling Standardbred geldings has been estimated at 18 and 26 min, respectively, associated with a mean endogenous basal secretion of 0.012 ± 0.014 µg/L/min.23 There is some evidence in horses that a single bout of exercise causes an increase in plasma GH concentration24,25,26,27,28 although the majority of these studies used heterologous GH assays. In a report based on a homologous GH assay, maximal treadmill exercise (comprising 2 min intervals at 30, 50, and 70% VO2max then to fatigue at 100% VO2max) in Standardbred geldings increased plasma GH transiently from 0.51 ± 0.076 to 8.43 ± 3.36 µg/L with normal secretion restored within 24 h.27 In contrast, nocturnal GH pulsatility was not affected by 18 weeks of moderate-intensity training in young Standardbreds preceded by 4 weeks of acclimatization to exercise, as determined by deconvolution analysis.29 On the other hand, overtraining in young Standardbreds following 24 weeks of moderate-intensity training was associated with an increase in concentration peak number (3.6 versus 2.0), a smaller peak secretion pattern with a prolonged GH half-life (15.2 versus 7.3 min), and an increased irregularity of nocturnal GH pulsatility pattern (as reflected by approximate entropy 0.89 versus 0.49) compared to control horses. As mentioned before, the amplitude of GH pulses is modulated by somatostatin resulting in a pattern of volleys of GH-secretory pulses with intervening periods of relative secretory quiescence. The observed absence of relative secretory quiescence suggests (relative) somatostatin deficiency. Four weeks of detraining did not lead to full recovery of the nocturnal GH pulsatility pattern (see Fig. 34.1). However, as basal GH secretion was not affected20b the presence of absolute GH deficiency is unlikely in overtrained horses. The fact that GH administration did not change aerobic capacity in young Standardbreds in exercise training also does not support the presence of absolute GH deficiency.30a In horses at rest, mean IGF-I concentration was 34.2 ± 13.6 nmol/L. In post-race samples (Thoroughbreds and Standardbreds mixed without specified racing conditions), IGF-I mean concentration was 24.5 ± 13.1 nmol/L, indicating that exercise does not affect plasma IGF-I concentration.30b Based on computerized algorithms it has been shown that there is a linear relationship between the magnitude of the acute increase in GH release and exercise intensity in human athletes.31 GH and its primary downstream mediator, IGF-I, play a critical role in formation, maintenance, and regeneration of skeletal muscles31,32,33,34 although it is not anabolic toward the contractile (i.e. myofibrillar) muscle tissue in healthy humans.35 However, it remains to be clarified whether skeletal muscle hypertrophy after exercise is stimulated primarily by endocrine or paracrine/autocrine IGF-I.32 The maintenance of normal blood glucose concentrations at rest and during exercise is critical and depends on the coordination and integration of several physiological systems, including the sympathetic nervous system and the endocrine system.36 Besides being the most important energy substrate for the central nervous system, glucose has a very important role in providing energy for muscular contraction. In horses, muscle glycogen, and to a lesser extent plasma glucose, are utilized in substantial amounts during low-, moderate- and high-intensity exercise because of stimulation of hepatic glycogenolysis, due to sympathoadrenergic mechanisms operating directly via hepatic sympathetic innervation or indirectly via circulating adrenaline37,38 (see also Chapter 33). Although carbohydrate availability to skeletal muscle affects maximal exercise performance in humans, this relationship is not very well described in horses.39 Glucose utilization and production are regulated in part by insulin.40 Glucose tolerance has been long studied in the equine species.41 During an oral glucose tolerance test (OGTT), the normal response to the absorption of the carbohydrate load is both to suppress hepatic glucose output and to enhance glucose uptake in muscle and liver. This requires a prompt increase in insulin secretion as well as adequate hepatic and muscle sensitivity to insulin. In particular, impaired fasting glycemia (or failure of plasma glucose concentration to decrease in response to fasting) is associated with decreased peripheral insulin sensitivity (IS), most importantly at the level of skeletal muscle (being the main site of glucose disposal). In human medicine, neither impaired glucose tolerance nor impaired fasting glycemia are considered clinical entities in their own right; rather they are risk factors for diabetes mellitus and represent metabolic states intermediate between normal glucose homeostasis and diabetic hyperglycemia.40 It is worth stressing that impaired glucose tolerance and impaired fasting glycemia are not interchangeable and that they represent metabolically distinct abnormalities with a different prevalence in humans.42a,42b,43 Impaired fasting glycemia has also been associated with decreased peripheral IS in the equine species (5.9 ± 1.4 in PPID and 4.9 ± 0.3 mmol/L in controls).44 The OGTT reflects the efficiency of the body to dispose of glucose after an oral glucose load. Thus, the OGTT provides useful information about glucose tolerance, but not insulin sensitivity/resistance per se.43 Insulin is a peptide hormone essential for appropriate tissue development, growth, and maintenance of whole-body glucose homeostasis by regulating carbohydrate, lipid and protein metabolism.45 It suppresses hepatic glucose and triglyceride production, inhibits adipose tissue lipolysis and whole-body and muscle proteolysis, and stimulates glucose uptake in muscle.45 These effects of insulin serve to promote the synthesis of carbohydrate, fat and protein; therefore, insulin is considered to be an anabolic hormone.46 As a protein hormone, insulin is rather species specific, is not lipophylic, and binds to a specific cell membrane receptor (see Fig. 34.2). Insulin is secreted by the β-cells of the pancreatic islets of Langerhans predominantly in response to increased circulating levels of glucose and amino acids.46,47 The early insulin response in horses was similar to that reported in other species, both during an IV glucose challenge49 and a standardized exercise test.50 A decline in plasma insulin is important for the rise in glucose production during exercise in a variety of species. In horses, as in humans and other species, there is suppression of insulin secretion during exercise. This suppression appears to have a threshold of 50% of VO2max.51 β-blockade (propranolol at 0.22 mg/kg BW IV) lowered the exercise-associated plasma insulin levels during consecutive 30-min bouts of low and moderate exercise in mixed-breed, conditioned horses compared to no change over time without treatment.38 In other reports, plasma insulin concentrations decreased by 35% following an incremental exercise test in untrained Standardbred mares52 and in horses competing in a 160 km endurance ride.53 Although decreased blood glucose concentrations did not occur in all horses following an 80 km endurance ride, insulin concentrations also fell in all horses examined associated with increased plasma concentrations of both adrenaline and noradrenaline post ride.54 However, rather than studying the relationship between exercise or training and plasma insulin concentration it is more appropriate to assess effects on IS itself. During prolonged exercise, increased demand for glucose by contracting muscle is in part met by an increase in glucose transport into muscle via glucose transporter-4 (GLUT4) proteins.55 Both insulin and muscle contraction increase the translocation of GLUT4 from intracellular sites to the plasma membrane (see Fig. 34.2) and transverse tubules, albeit by activation of different signaling pathways. This perhaps explains why in humans with IR, an acute bout of exercise but not insulin results in increased muscle glucose uptake.55,56 Furthermore, muscle contractions not only stimulate muscle glucose uptake independent of insulin but also increase IS. The contraction signaling pathway does not increase insulin receptor kinase or PI 3-kinase activity. However, the possibility that two distinct GLUT4 pools may be targeted, one by insulin and the other by contractions, cannot be excluded.57 Unlike insulin-stimulated glucose transport, in which permeability is reversed rapidly upon removal of the insulin, the increase in membrane permeability following contractile activity can persist for many hours in human athletes.56 Similarly to other species, the equine GLUT4 cDNA encodes 509 amino acids. The calculated molecular weight of the resulting protein is 54.8 kDa.39 In equine skeletal muscle, GLUT4 is expressed in a fiber type selective manner predominantly expressed in the cytosol of fast-type 2B as shown in vastus lateralis fibers of Warmblood horses.58 GLUT4 translocation in semitendinosus muscle of Shetland ponies is insulin-dependent as has been described in other species,59 although active cell-surface GLUT4 protein content might not have been assessed optimally in this study, possibly explaining the reported poor responsiveness to insulin.60 The effects of exercise training on skeletal muscle GLUT4 content are similar to those found in other mammals and man. Prolonged, submaximal exercise61,62 and short periods of strenuous training39,63 increased skeletal muscle GLUT4 protein and mRNA content in horses, but single bouts of intense exercise had no significant effect on equine skeletal muscle GLUT4 protein61,64 and mRNA expression.65 Insulin resistance selectively decreased active basal cell-surface GLUT4 in equine semimembranosus muscle, without altering total GLUT4 content and further enhancement of cell-surface GLUTs after in vitro insulin stimulation. The observed lack of substantial increase in insulin-stimulated GLUT4 translocation in healthy insulin-sensitive horses may be the result of the insulin-dependent signaling pathways regulating GLUTs already being at near maximal physiologic limits for the species.60 In addition, a single bout of moderate-intensity exercise in Standardbred horses increased the glucose disposal under hyperinsulinemic conditions (5.6 ± 1.1 versus 3.3 ± 0.9 mg/kg BW/min in controls) without enhanced non-insulin-mediated glucose uptake post-exercise66 perhaps suggesting that the insulin-stimulated glucose transport by far exceeds the increase in membrane permeability following contractile activity. Insulin has concentration-dependent saturable actions to increase whole-body glucose disposal. The maximal effect of insulin defines ‘insulin responsiveness’, whereas the insulin concentration required for a half-maximal response defines ‘insulin sensitivity’.42a IS is the reciprocal of IR.40 IR is associated with defective signal transduction.45 The phenomenon of IR can be illustrated by showing the presence of impaired glucose tolerance and/or impaired fasting glycemia.40 Of interest, neurons can develop hyperinsulinemia-induced IR probably pathogenic in common neurological disorders such as diabetic neuropathy.67 Direct and indirect methods of varying complexity are currently employed for assessing peripheral insulin sensitivity (IS), with the euglycemic-hyperinsulinemic clamp (EHC) technique being widely accepted as the gold standard for direct determination of IS.42a,43,68,69 In the classical glucose tolerance tests, the dose of glucose is fixed, and the measure of tolerance is the plasma glucose concentration. In the clamp techniques, the plasma glucose concentration is fixed68 and the glucose administered becomes the measure of insulin sensitivity.70,71,72,73,74 The direct methods comprise the EHC and the insulin suppression test (IST) and the indirect methods include the classical glucose tolerance tests. Besides, there are simple surrogate indexes for IR derived from fasting steady-state conditions.42b Two glucose clamp techniques have been validated for use in horses, namely the hyperglycemic clamp test and the EHC.66,71,72,73,74 The former allows quantification of the sensitivity of β-cells to glucose and the EHC allows quantification of the sensitivity of tissues to insulin. In addition, the principle of both clamp techniques is that the rate of glucose infusion required to maintain a steady state is an index of glucose metabolism and as a result they both can be used to quantify the rate of glucose disposal.68 In equine medicine, clamping of blood glucose during the EHC is usually within the normal range (euglycemic) in contrast to fasting levels (isoglycemic). The IST (using somatostatin or a somatostatin analogue to suppress endogenous secretion of insulin and glucagon combined with simultaneous glucose and insulin infusions) also directly assesses IR.43 To the author’s knowledge, the IST has not been used in horses yet. However, in Standardbred horses it has been shown that infusion of somatostatin (500 µg as a bolus followed by a constant-rate infusion of 1 µg/kg BW/h for 150 min) indeed suppressed endogenous insulin secretion.66 Many of the limitations of the IST are similar to those described for the EHC, with the exception that the IST is less technically demanding.43 The minimal model provides an indirect measurement of insulin sensitivity/resistance on the basis of glucose and insulin data obtained during a 3-hour frequently sampled intravenous glucose tolerance test (FSIVGTT).74,75,76 Blood samples are taken for plasma glucose and insulin measurements and subjected to minimal model analysis using software to generate the index of insulin sensitivity (Si) among others.74 The minimal model is defined by two coupled differential equations (plasma glucose dynamics in a single compartment and insulin dynamics in a remote compartment).77,78 It is a physiologic compartmental representation of the classical glucose tolerance tests and includes 4 indices (the action of insulin on glucose uptake (Si); the capacity of tissues for glucose uptake independent of insulin stimulation (glucose effectiveness, i.e. Sg); the AIRg provides a measure of insulin secretion during the first 10 min after glucose injection, whereas disposition index (a product of Si and AIRg) reflects the combined effect of these two factors (insulin efficiency and insulin secretion) to minimize hyperglycaemia).78 In addition, a modified frequently sampled intravenous glucose tolerance test (CGIT) has been proposed where exogenous insulin (0.03–0.1 IU/kg BW) is also administered, beginning immediately after the intravenous glucose bolus. Blood samples are taken for plasma glucose and insulin measurements.79 Limitations of minimal model analysis stem from the fact that the model oversimplifies the physiology of glucose homeostasis. Another oversimplification involves lumping together effects of insulin to promote peripheral glucose utilization and suppress hepatic glucose production. The non-systematic errors are highlighted by calibration model analysis.80 Since the minimal model relies on a dynamic test to evaluate IS, estimates of the action of insulin on glucose uptake (Si) are much less reliable in individuals with impaired insulin secretion and/or significant IR.43,81,82,83,84 In addition, a study of the repeatability of both the minimal model and the EHC found that the latter technique provided more closely repeatable results in the equine species.74 Caveats should be noted regarding limitations of the minimal model and the availability of simpler, more accurate methods for assessing IS.43 The minimal model was originally developed in dogs, which have a delayed insulin secretory response to the FSIVGTT when compared with humans.84 To increase the accuracy of Si in humans, a modified FSIVGTT was developed where exogenous insulin (or tolbutamide) is given at a time where glucose levels have decreased to near-normal levels (beginning 20 min after the intravenous glucose bolus), thereby allowing the minimal model to more effectively distinguish between glucose effectiveness and IS.42a In the equine species, the early insulin response is fast and similar to humans, as mentioned earlier. However, the possibility of hypoglycemia and ensuing counter-regulatory homeostatic mechanisms have the potential to further confound estimates of IS. The CGIT also has limitations including suboptimal repeatability,79 especially regarding the negative phase glucose clearance (CV 33%).85 In healthy humans, the fasting condition represents a basal steady state where glucose is homeostatically maintained in the normal range such that insulin levels are not significantly changing and hepatic glucose production is constant.43 Simple surrogate indexes are inexpensive quantitative tools that can be easily applied in almost every setting.42a A critical condition and assumption of simple surrogate indexes is that subjects are strictly fasting and in a basal steady-state condition with respect to glycemia, insulinemia, and hepatic glucose production. Surrogate indexes based on fasting glucose and insulin concentrations reflect primarily hepatic insulin sensitivity/resistance. However, under most conditions, hepatic and skeletal muscle insulin sensitivity/resistance are proportional to each other.43 Validation of these surrogates against glucose clamp measurements has not been extensively conducted in animals like horses. QUICKI appears to be the best proxy in human insulin-resistant subjects, whereas Si from the minimal model performs best in healthy, insulin-sensitive subjects,42a,42b with the glucose clamp technique being widely accepted as the gold standard for directly determining IS in healthy and diseased (IR) human subjects.43,68,69 Further study is needed to determine whether the same is true for horses. Physical activity has a beneficial effect on IS in normal as well as IR human populations. A distinction should be made between the acute effects of exercise and genuine training effects. Up to 2 hours after exercise, glucose uptake is in part elevated due to insulin-independent mechanisms, probably involving a contraction-induced increase in the amount of GLUT4 associated with the plasma membrane and T-tubules. There is robust evidence in healthy human subjects that a single bout of exercise causes an increase in IS to glucose disposal and this effect has been reported to persist for a period ranging from 2–48 h post-exercise.86,87 In contrast, no increase in IS was measured 0.5–24 hours after a single bout of moderate exercise in trained Standardbred horses by using EHC.64 On the other hand, short-term (7-day) training increased IS 24 hours after the final exercise in untrained aged lean and obese mares71 and in untrained Standardbreds from 18 ± 4 to 44 ± 6 µg/kg BW/min per mu/mL.62 The approximately 2-fold increase in whole-body IS measured during an EHC was associated with an over 10-fold increase in skeletal muscle GLUT4 protein content and these enhancements persisted after 5 days of inactivity with IS still 1.6 times larger than basal.62 IS returned to pre-training values by 9 days post-training in both the obese and lean mares, suggesting that transient improvement in IS occurred in obese mares already following a short period of training and in the absence of a necessary change in bodyweight. Interestingly, a greater increase in IS in lean, more-insulin sensitive horses compared to obese, less insulin-sensitive horses was observed 24 h post training by use of the EHC test.71 In untrained overweight or obese, insulin-resistant horses, 8 weeks of low- to moderate-intensity training without dietary restriction did not alter IS, as assessed by minimal model analysis.88 Although minimal model analysis might be not most appropriate in insulin-resistant horses, similar results were found following 18 weeks of moderate-intensity training in young Standardbreds preceded by 4 weeks of acclimatization to exercise by means of the EHC.29 Of interest, during low-intensity endurance exercise in exercise-trained Arabian geldings, markedly increased IS has been measured. Values increased approximately 12-fold in fat- and fibre-fed horses and 7-fold in starch- and sugar-fed horses as assessed by minimal model analysis.89 It might be concluded from the previous findings that minimal training-associated improvement in IS occurs in horses, perhaps due to the possibility of the insulin-dependent signaling pathways regulating GLUTs already being at near-maximal physiologic limits in contrast to findings in healthy trained human subjects.56 It should also be noted that improved IS after a period of physical conditioning might be due to weight loss rather than training itself.90 IR of skeletal muscle glucose transport is a key defect in the development of impaired glucose tolerance and type 2 diabetes and it is well established in humans and rodents that both an acute bout of exercise and chronic endurance training can have beneficial effects on insulin action in insulin-resistant states. This training-induced enhancement of insulin action is associated with upregulation of specific components of the glucose transport system in IR muscle and includes increased protein expression of GLUT4 and insulin receptor substrate-1.91 Consequently, physical training can be considered to play an important, if not essential, role in the treatment and prevention of insulin resistance.86
Endocrine function during exercise and response to training
Introduction
Growth hormone
Major actions of growth hormone
Regulation of GH secretion
Effects of exercise and training on secretion and circulating GH concentrations
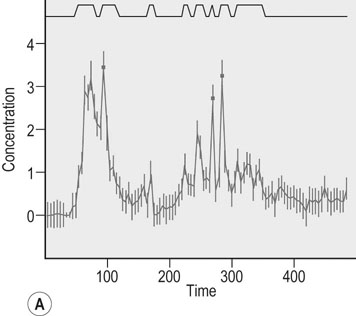
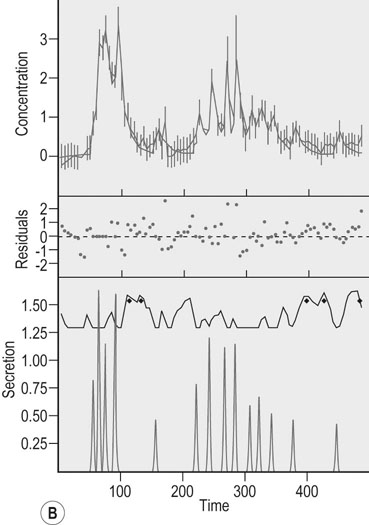
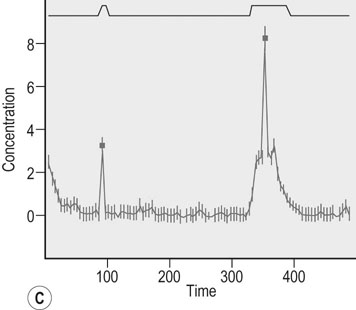
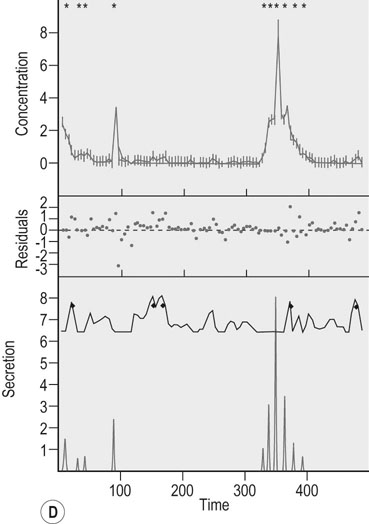
Actions of growth hormone during exercise
Glucose tolerance
Insulin
Major actions of insulin
Regulation of insulin secretion
Effects of exercise and training on secretion and circulating insulin concentrations
Actions during exercise and training
Insulin sensitivity and resistance
Assessment of insulin sensitivity
Effects of exercise and physical conditioning on insulin sensitivity
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine function during exercise and response to training
