CHAPTER 39Endocrine Diagnostics and Therapeutics for the Stallion with Declining Fertility
HORMONE REGULATION OF REPRODUCTIVE FUNCTION IN THE STALLION
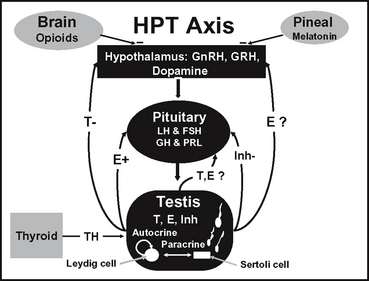
In most mammalian male species including the stallion, normal reproductive function is dependent upon a dynamic and integrative hypothalamic-pituitary-testicular axis (HPT) involving the classic hormone actions of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle stimulating hormone (FSH), and the feedback mechanisms of steroids (androgens and estrogens) and proteins (inhibin and activin).1–3 In the last decade it has become evident that other hormones such as prolactin, growth hormone (GH), thyroid hormone, vasopressin, opioids, and oxytocin regulate testicular function via the pituitary-testicular axis.4,5 In addition, local testicular factors called paracrine/autocrine factors, such as growth factors, cytokines, and GnRH-like peptides, appear to modulate testicular cell function.3,4,6 The presence of these local factors and the dynamic interactions with the endocrine system and fertility in the stallion are under investigation.3 Specific endocrine tests measuring some of the above hormones are available as diagnostic tools for stallions presented with declining fertility,7,8 but very few tests are available to measure paracrine/autocrine factors unless a testicular biopsy can be obtained so factors can be extracted from the tissue.8 When an idiopathic subfertile stallion with testicular dysfunction and abnormal endocrine parameters is presented to a clinician, the first question asked is whether there are any endocrine therapies to prevent a further decline in spermatogenesis or to reverse the existing problem. This chapter presents an overview of the endocrine and possible paracrine and autocrine systems that regulate normal spermatogenesis in the stallion, the endocrine and possible paracrine and autocrine dynamics in idiopathic subfertile/infertile stallion, endocrine diagnostics for idiopathic subfertile/infertile stallions, and potential endocrine therapies.
Endocrine/Paracrine/Autocrine Systems
Endocrine System
The dynamic nature of the hormone interactions of the pineal gland and the HPT axis is constantly evolving (Figure 39-1). Studies in the stallion and other species demonstrate that hormones and local factors not previously considered to play a role in reproductive function may in fact be involved.3,9–12 Since reviews on the classic endocrine system of the HPT axis in the stallion have been previously published,1,3,13 only a brief discussion with updates will be presented herein.
Pineal gland and melatonin.
The classic reproductive endocrine system in a seasonal breeder, such as the stallion, starts at the level of the pineal gland and the secretion of melatonin.14 Melatonin is controlled by light signals received by the retina and transmitted via the optic nerve to the pineal gland.14 With decreasing light, the pineal gland produces more melatonin, which inhibits the release of GnRH, resulting in a decrease in the gonadotropins, steroids, and testicular activity.14 The endocrine events leading to maximum reproductive capacity in the stallion are initiated when day lengths are still short, albeit increasing.15 The increase in day length (or light) is part of an external environmental rhythm or zeitgaber (time-giver) needed to stimulate recrudescence.15 The fact that the stallion has an endogenous circannual cycle entrained by a zeitgeber should be kept in mind when sending breeding stallions from the northern to southern hemisphere. Without a period of short days (2-3 months) followed by increasing light, libido and sperm production could decline over time, particularly if the stallion is already a marginal breeder. Increasing the period of light suppresses melatonin synthesis by modulating the activity of its biosynthetic enzymes16 and in turn, GnRH, LH, and FSH are released and testicular activity resumes.17 Cleaver et al18 reported that exposure of mares to constant light resulted in a marked decrease in circulating melatonin concentrations and an increase in hypothalamic GnRH content. It has been demonstrated that exogenous melatonin decreases plasma testosterone concentrations in stallions.19
HPT axis and feedback mechanisms.
During the breeding season, when melatonin is low in seasonal breeders including the stallion, GnRH, in a pulsatile fashion, stimulates the gonadotroph cells in the anterior pituitary to produce LH and FSH.20,21 These 2 gonadotropins are glycoproteins that are composed of 2 subunits, an α and β. The gonadotropins share the same α subunit but have different β subunits that designate the specificity of the hormone.22 In the stallion, LH and FSH are episodically secreted23,24 into the peripheral circulation where they travel to the testes to stimulate steroidogenesis and other actions in Leydig and Sertoli cells, respectively.25,26 In the adult stallion, LH is responsible for stimulating androgens and estrogens from the Leydig cell,25 whereas FSH stimulates estrogen, insulin-like growth factor (IGF-1), and transferrin from Sertoli cells.3,26,27 It has been documented that FSH stimulates inhibin production from Sertoli cells in large domestic species,28 and that inhibin is produced by the equine testis, specifically the Sertoli cells.29,30 Studies are ongoing to show that FSH stimulates inhibin production from equine Sertoli cells.
A closed loop feedback system regulates the amounts of GnRH, LH, and FSH released from the hypothalamus and pituitary, respectively. This feedback system involves steroids and proteins from the testis in most mammalian species including the stallion.1,13,31 In the stallion it has been shown that the major feedback mechanism of testosterone appears to be at the level of the hypothalamus on GnRH to suppress LH.32,33 In contrast, estradiol appears to increase the GnRH-induced LH release from the pituitary32 and decrease baseline levels of FSH in serum.33 The feedback effect of inhibin appears to be at the level of the pituitary on inhibiting FSH.34 In addition to these classic reproductive hormones, other hormones such as prolactin, thyroid hormone, GH, and opioids have been investigated in the horse to determine if they play a role in reproductive function.
Prolactin, thyroid hormone, and GH.
Prolactin, thyroid hormone, and GH have been reported to effect testicular function in other species. Prolactin appears to play a role in inducing transcription of the estrogen receptor.35 Thyroid hormone in the adult rat is crucial to the onset of adult Leydig cell differentiation.36 GH resistance is associated with reduced fertility in men.37 GH may alter gametogenesis by affecting testosterone synthesis since testosterone is necessary for sperm production. GH increases basal or hCG-stimulated testosterone production in GH-deficient men.38 Although prolactin and thyroid hormone fluctuate with the season in the horse,39,40 the role of these hormones in reproductive function in the stallion is yet to be determined. Prolactin did not appear to stimulate testosterone production in equine Leydig cells in culture,25 and the effects of thyroid hormone have not been investigated in the stallion. In the mare, thyroid function was not associated with infertility.41 GH does not appear to fluctuate with season in the stallion.42 However, treatment with a somatostatin analogue (GH inhibitor) caused a transient decrease in semen motility and hCG-induced testosterone release, suggesting a role of GH in the regulation of testicular function in stallions.42 A direct effect of GH on Leydig cell steroidogenesis in culture was not observed.43
Opioids.
In the stallion as well as other species, it appears that seasonal changes in GnRH/LH release are partly regulated by opioids. Opioids, secreted from the brain, travel to the hypothalamus and cause a decrease in GnRH release during the winter months.44 Aurich et al45 showed that treatment with an opioid antagonist naloxone caused an acute LH release in stallions outside of but not during the breeding season. The opioidioidergic regulation of LH release appears to require the presence of the gonads.46
Paracrine/Autocrine System
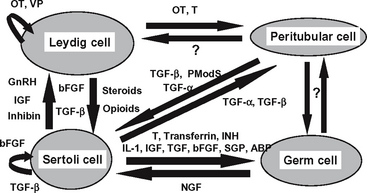
Although endocrine regulation of the testes is important to ensure that reproductive processes are synchronized with physiologic events, it has become increasingly apparent that it is probably the local paracrine/autocrine system that modulates the intricate network of cellular interactions in the stallion (Figure 39-2).3 The roles of the paracrine and autocrine systems are to coordinate local functions between and within the 2 testicular compartments by modulating testicular actions of the endocrine system.47 Paracrine factors are produced by one cell type and act on another cell type present in the same organ. Autocrine factors are substances that are produced by one cell type that act back on the same cell type.47 Only a few of the relatively large group of paracrine/autocrine factors have been identified in the stallion testis: steroids, inhibin, IGF-I, and transferrin. IGF-I has been identified in testicular tissue in the stallion,48 particularly in the Leydig and Sertoli cells.3 Highest IGF-I production rates in Leydig cells appear to be during puberty, whereas highest production rates in Sertoli cells occur prior to puberty.3 Although LH and GH do not appear to regulate IGF-I in Leydig cells, FSH does appear to stimulate IGF-I from Sertoli cells, but only prior to puberty.3 However, IGF-I itself does not appear to stimulate a steroidogenic response from Leydig cells.43
In the stallion, transferrin, which has been postulated to deliver iron to developing germ cells,49 is produced in Sertoli cells, with the highest production rate occurring at the time of puberty.27 The levels of transferrin are proportional to sperm production in humans and cattle and may be an effective indicator of Sertoli cell function.50
Sertoli cells produce estrogen, with the highest production rate occurring during puberty.51 Leydig cells appear to produce most of the estrogen in the adult stallion.43,52 Testosterone is produced by the Leydig cells throughout development and into adulthood.43 Recent evidence from our laboratory indicates that estrogen receptors are located on Leydig, Sertoli, and germ cells, depending on the stage of sexual development (personal observations). Androgen receptors are located on Sertoli, peritubular, and Leydig cells throughout development (personal observations). These data suggest that both estrogens and androgens are important locally in early testicular development and spermatogenesis in the stallion. Androgens have been reported to play a crucial role in the development of male reproductive organs and spermatogenesis.53 In the last decade it has been demonstrated that estrogen is also important in regulating testicular function in the male.54 Of interest is that estrogen has been reported to regulate inhibin production.55 When stallions were given an aromatase inhibitor to block estrogen production, inhibin levels decreased while FSH levels remained the same,56 suggesting a local control of inhibin by estrogen. In other species it has been demonstrated that inhibin acts as a paracrine factor influencing Leydig cell steroidogenesis,57–59 as well as spermatogenesis.60,61
The Endocrine, Paracrine, and Autocrine Dynamics in Idiopathic Subfertile/Infertile Stallions
Historically clinicians relied on baseline concentrations of reproductive hormones in the stallion to help diagnose breeding unsoundness. For example, low levels of testosterone were observed in azoospermic stallions.62 Burns and Douglas63 made the first association between elevated plasma concentrations of FSH and subfertility in stallions. Two studies indicated that circulating plasma levels of FSH and estrogens, and not LH and testosterone, could be good markers to predict future changes in fertility,7,64 whereas another group of investigators suggested that measurement of serum levels of estrogens and testosterone may help in the diagnosis of infertile stallions when other methods are not available.65 Whitcomb and coworkers66 presented interesting data on the presence of what appears to be a circulating bioinactive LH isoform in stallions with poor breeding performance.
In addition to measuring basal levels of reproductive hormones in stallions with poor fertility, our laboratory has used the GnRH challenge test, whereby a bolus or intermittent infusion doses of synthetic GnRH were given to diagnostically assess pituitary and testicular responsiveness.64,67 In human medicine, the GnRH challenge test appears to be of some value in revealing intrinsic differences in acute gonadotroph and Leydig cell stimulation between normal men and men with reproductive disorders.68,69 When stallions were challenged with repetitive pulses of exogenous GnRH, different pituitary and testicular responses were observed between fertile and subfertile stallions and appeared to be dependent on season and gonadal status.64,70 The pituitary and testes of fertile stallions appeared to be more responsive to pulsatile administration of GnRH in the nonbreeding season than the breeding season.70 In contrast, the pituitary responsiveness to repetitive pulses of GnRH in subfertile stallions was significantly reduced during the nonbreeding season and not affected by seasonal changes.70 When stallions were subject to a discrete dose of GnRH in a subsequent study, responsiveness was observed to be normal,67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree