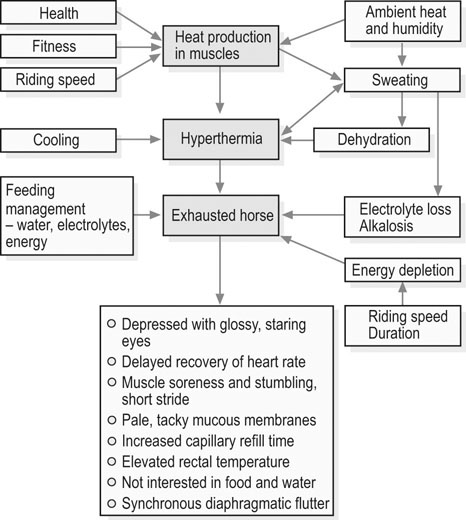
Unlike their sedentary or aged counterparts, endocrine and metabolic disorders of the athletic horse are relatively rare. However, when horses are excessively exercised without the appropriate acclimation or conditioning, or when horses are chronically overtrained, marked metabolic and endocrine derangements can occur. This chapter describes two metabolic/endocrine conditions of athletic horses: the exhausted horse and overtraining syndromes. Synchronous diaphragmatic flutter, which can occur in exhausted horses, is described elsewhere in this book (Chapter 40). • This is a multisystemic condition due to the combined effects of dehydration, hypovolemia, acid–base and electrolyte disturbances, energy substrate depletion, and hyperthermia. • Clinical signs include depression, lack of impulsion and shortened stride, muscle fasciculations and soreness, increased capillary and jugular refill times, hyperthermia, delayed recovery of heart rate, and reduced or absent borborygmi. • Treatment focuses on correction of fluid and electrolyte deficits and aggressive cooling of the overheated horse. • Despite extensive supportive care, severely affected horses may die. The exhausted horse syndrome is a term used to describe a multisystem disorder that can develop in horses participating in athletic activity over an extended period (e.g. three-day events, endurance racing, and other long-distance rides), particularly when exercise is performed in hot ambient conditions.1–3 The clinical presentation is variable and dependent upon the severity and speed of onset of fatigue as well as the individual horse’s tolerance to physiological strain. In general, clinical signs are reflective of dehydration, electrolyte and acid–base disturbances, depletion of energy reserves, hyperthermia and muscle pain.2,3 Initially, there are subtle signs of fatigue with mild alterations in mental state (alertness and attitude) and inconsistencies in gait that may be due to muscle soreness. Clinical evidence of exhaustion becomes more pronounced if exercise is continued; muscles start to show localized hardening and pain on palpation, the gait becomes stiff and stilted, and muscle spasms and cramps may occur, as may synchronous diaphragmatic flutter (SDF).2,3 Severely affected horses are often unwilling to continue to exercise, are depressed and are unwilling to eat or drink. They may show incoordination (ataxia), become recumbent, and show other neurologic signs such as head pressing and seizures. Dehydration is expressed clinically as decreased skin turgor, sunken eyes, dry mucous membranes, firm, dry feces and decreased urine output. Mild signs of dehydration usually become apparent at a body water deficit of 4–5%. Subjectively, the sweating response may appear inappropriate relative to the level of hyperthermia (e.g. a patchy appearance or, in severe cases, a hot and dry coat). Despite significant dehydration, affected horses often are not interested in water or feed. Intestinal stasis commonly occurs with decreased or absent borborygmi, poor anal tone and, occasionally, colic.2,3 In an exhausted horse, early clinicopathologic findings include increased packed cell volume (PCV) and plasma protein concentration due to dehydration, normo- or slight hyponatremia, hypokalemia, hypocalcemia (ionized), hypochloremia, and azotemia.1–3 The PCV of endurance horses eliminated from races due to metabolic problems may be as high as 55–65%, while plasma protein concentration may exceed 10 g/dl (typical reference range, 5.9 to 7.5 g/dl). As a result of muscle exertion or damage, increases in muscle enzyme activity (e.g. creatine kinase, CK; and aspartate amino transferase, AST) and plasma phosphorus concentration may be evident. Plasma creatinine values may be elevated, suggesting a reduction in glomerular filtration rate because of dehydration. Urine samples appear dark due to myoglobinuria, hematuria, proteinuria and glycosuria.4,5 Serum electrolyte concentrations may be decreased (especially, K+, Cl−, Ca2+ and Mg2+). Metabolic alkalosis is the most common acid–base disturbance although metabolic acidosis may develop in horses with profound hypovolaemia and circulatory shock.6 Metabolic alkalosis along with hypocalcemia and hypokalemia contribute to the development of SDF. Profound neutropenia with a left shift is sometimes observed within 24 h of the onset of exhaustion.3 It should be noted that laboratory evidence of muscle damage as well as disturbances in electrolyte and acid–base balance also may be present in horses that successfully complete endurance races. Conversely, some exhausted horses may not show substantial alterations in clinicopathological variables at the time of diagnosis. During endurance races, muscle enzyme activities and serum creatinine concentration increase as a function of distance covered and in one study were not useful for the early diagnosis of metabolic stress.7 For mildly affected horses, rest together with cooling out and access to water, salt and feed may be sufficient. However, fluid therapy is required if the horse does not drink or eat within 15–30 minutes. In severe cases, fluid therapy should be started immediately. Under most circumstances, it is better to treat a horse in the field and not to attempt a trailer ride until it has been rehydrated. Oral fluid therapy may be considered in mildly affected horses that have adequate gut sounds and no evidence of colic. Oral fluid administration offers the advantages of speed and convenience and can be used if the horse has normal gut sounds. Between 4–8 L of fluid can be given via nasogastric tube every 30–60 min until the horse shows signs of improvement. Oral administration can be started immediately after exercise, because consumption of 10–15 L of cool (~16°C) water within 3–5 min of completing exercise is not harmful.9,10 Isotonic solutions containing sodium, potassium, calcium, chloride and glucose are often well tolerated and fairly rapidly absorbed. Commercial electrolyte powders should be those formulated for horses. For example, electrolyte formulations designed for use in calves with diarrhea usually contain bicarbonate, lactate or citrate. Therefore, these preparations are not the best choice for the treatment of horses with exercise-induced dehydration because of their alkalinizing effects. The oral administration of hypertonic solutions should also be avoided because they may cause a transient reduction in plasma volume due to movement of water into the bowel lumen.9,11 Oral administration should be halted if any discomfort or gastric reflux becomes apparent. The use of hypertonic solutions (e.g. 1–2 L of a 7.5% NaCl solution) for resuscitation of severely dehydrated, hypovolemic horses is controversial due to concern that the administration of a hyperosmotic solution will exacerbate intracellular dehydration. In one study of endurance horses disqualified from a race for metabolic conditions, the administration of 2 L 7.2% NaCl in combination with 5 L of LRS solution resulted in greater decreases in PCV, total plasma protein, and albumin concentration and earlier onset of urine production when compared to treatment with LRS alone.12 It was concluded that the i.v. administration of hypertonic solutions appears to be safe in the treatment of dehydrated endurance horses but this treatment must always be accompanied by the administration of isotonic fluids for correction of fluid deficits.12 A thorough physical examination, including evaluation for gastric reflux, palpation per rectum, and abdominal ultrasonography, should be performed in horses with colic. Ileus appears to be primary cause of colic pain in exhausted horses and the colic often resolves with fluid therapy and an improvement in intestinal motility. Pain should be managed with xylazine (0.33 to 0.55 mg/kg i.v.), butorphanol (0.01 to 0.02 mg/kg i.v.), or a combination of the two. Surgical lesions (e.g. small intestinal volvulus) have been reported in endurance horses after racing;3 repeated examinations are therefore imperative for horses that show persistent colic in the face of medical treatment. Exhaustion occurs when the horse is required to exercise beyond its physiological limit, with the combined effects of dehydration, hypovolemia, electrolyte loss, acid–base disturbances, energy substrate depletion, and hyperthermia contributing to the pathophysiology of exhaustion and its sequelae (Fig. 35.1). Risk of exhaustion is greatest when horses compete under conditions of high heat and humidity, especially in under-conditioned animals or those not properly acclimated to exercise in hot ambient conditions. Lameness also may contribute to the development of exhaustion by altering a horse’s gait, leading to increased use of certain muscle groups and earlier onset of fatigue. Depletion of energy stores in endurance horses, especially during 100 km or longer rides,13 may contribute to the onset of exhaustion. Dehydration and a decrease in effective circulating volume occur as a result of sweat fluid losses (and the failure to replenish at least some of these losses during performance). Physical exercise leads to considerable heat production, because only 20 to 25% of the energy utilized in muscles is converted to mechanical energy. Evaporation of sweat is the most efficient means of heat loss during exercise and may be the only means of heat dissipation in a hot environment. The amount of sweat produced depends on the horse’s size and fitness, on work intensity and the environmental conditions. Under cool climatic conditions, horses may sweat 5 to 8 L/h but in hot weather sweat production may amount to 10 to 15 L/h when activity levels are maintained at a high rate (e.g. 15 km/h). During endurance competitions, horses routinely lose 4 to 7% of their bodyweight but in hot conditions, net water loss may be about 40 L or close to 10% of bodyweight.14 Respective values during the endurance phase of a three-day event range from 2% to 4% of bodyweight under normal conditions15 and in hot conditions deficits greater than 9% of bodyweight have been reported.16 During sweating, water is mainly lost from the extracellular fluid and the consequent decreases in blood and plasma volumes1,11,17 can reduce perfusion in skeletal muscle and in other vital organs. Inadequate tissue perfusion leads to inefficient oxygen and substrate transport, and hampers thermoregulation. If severe, this cardiovascular compromise may contribute to impaired renal and intestinal function. Sweating-induced dehydration is always accompanied by electrolyte loss. Equine sweat is isotonic or slightly hypertonic relative to plasma and contains high concentrations of sodium, potassium and chloride and also some calcium and magnesium.10,18 Abundant sweating will incur significant ion deficits; these will lead to alterations in skeletal muscle ion content, increasing the potential for muscular dysfunction and contributing directly to fatigue.18 The most consistent acid–base alteration associated with endurance exercise in a hot environment is metabolic alkalosis.6 Endurance horses exercise at moderate work intensities at a fairly constant speed between 10 and 20 km/h and rely almost totally on aerobic energy metabolism, with minimal accumulation of lactate in blood. Typical plasma lactate concentrations during such exercise are 1.0 to 3.3 mmol/L.18 The degree of metabolic alkalosis is dependent on the severity of hypochloremia and hypokalemia. Hypochloremia is associated with an increase in plasma bicarbonate because in the kidney when chloride concentration is low, bicarbonate (HCO3−) is resorbed. As plasma sodium concentration tends to decrease due to loss in sweat, the kidney conserves sodium at the expense of potassium and hydrogen ions, which also contributes to the alkalosis.1,14 Potassium, magnesium, and calcium depletion associated with metabolic alkalosis may alter membrane potential and neuromuscular transmission, contributing to gastrointestinal stasis, cardiac arrhythmias and muscle cramps and spasms including SDF.14 The situation differs during the endurance phase of a three-day event and the marathon phase in combined driving, during which anaerobic metabolism significantly contributes to energy transduction, plasma lactate concentrations are very high (up to 38.5 to 40.2 mmol/L),6,19,20 and horses may develop metabolic acidosis. After exercise, the acidosis is resolved through oxidation of lactate during a 30-min to 2-h period. Thereafter, metabolic alkalosis prevails.
Endocrine and metabolic disorders of the equine athlete
Introduction
Exhausted horse syndrome
Recognition
History and presenting complaint
Physical examination
Laboratory examination
Treatment and prognosis
Therapy
Etiology and pathophysiology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine and metabolic disorders of the equine athlete
