Chapter 38 Emergency and Critical Care of Small Mammals
Cardiopulmonary-Cerebral Resuscitation
Principles
The ABCs (airway, breathing, and circulation) of human emergency medicine are universal and apply as well to the exotic companion mammal patient.21 The general goal of cardiopulmonary resuscitation is restoration of spontaneous circulation. In the 2000s, the American Heart Association changed the guidelines to include preservation of neurologic function as a goal of successful resuscitation. Therefore the term cardiopulmonary-cerebral resuscitation (CPCR) was adopted.2,33 Guidelines have been reviewed and modified for veterinary patients.8–10 Basic life support consists of the ABC approach, while advanced life support consists of electrocardiographic (ECG) identification of the abnormal rhythm, defibrillation, fluid and drug administration, and postresuscitative care.2
CPCR in Small Mammals: Respiratory Arrest
In the event of respiratory arrest, the ideal treatment is immediate endotracheal intubation. Intubation can be challenging in exotic companion mammals, as many are obligate or dependent nasal breathers with limited oral airway access.20 Intubation of the ferret and other exotic carnivores is straightforward, using direct visualization, similar to the technique used in the domestic cat. Rabbits may be intubated using the blind orotracheal or nasotracheal technique. An alternative for rabbits is otoscope- or endoscope-guided orotracheal intubation, using the endotracheal tube over the endoscope (over-the-top method) or side by side.20 Larger guinea pigs can also be intubated using this method.6,17 It must be emphasized that all intubation techniques require practice, and proficiency should be gained prior to presentation of a patient in respiratory arrest.
In reality, most smaller mammals and rodents are difficult to intubate without significant practice.5,20,38,40 Therefore the following options should be considered:
• Forced high-flow oxygen ventilation using a tight-fitting mask over the nose and mouth. Positive-pressure ventilation should be provided using 100% oxygen at a rate of 20 to 30 breaths per minute (Fig. 38-1). A disadvantage of this technique is accumulation of gastric air and bloat, which can limit movement of the diaphragm.
• Tracheostomy. This procedure is similar to that described in dogs and cats.7 Make a 2- to 3-cm skin incision on the ventral midline parallel to the trachea, just proximal to the larynx. Bluntly dissect the subcutaneous fat and fascia, taking care to avoid blood vessels within the fat. Blunt dissection is continued through the sternohyoid and sternothyroid muscles to isolate the trachea. Make a transverse incision between the tracheal rings, which should not exceed 50% of the circumference of the trachea. Place stay sutures in the trachea cranial and caudal to the tracheostomy site. Insert an endotracheal tube or cannula into the trachea and secure it in place.
CPCR in Small Mammals: Cardiac Arrest
Cardiac arrest is cessation of effective circulation and is recognized by loss of consciousness and collapse. A palpable pulse is absent, the mucous membranes are pale or cyanotic, and respirations commonly cease (i.e., cardiopulmonary arrest).2 Box 38-1 presents a CPCR flow chart for use in exotic companion mammals. Intubate the patient and ventilate with 100% oxygen or, alternatively, deliver forced high-flow oxygen as directed above. Chest compressions at the rate of 100 to 120 times per minute directly compress the myocardium, resulting in increased cardiac output. It is important that both hands be placed on both sides of the chest with compressions done at the widest portion of the chest. The duration of the compression should be half of the total compression-release cycle.2
Box 38-1 CPCR Protocol for Use in Small Mammals
| No respirations (respiratory arrest), but pulse present and heart rate present | No respirations (cardiac arrest), and no pulse and no heartbeat |
|---|---|
| Turn off anesthesia if applicable. | Turn off anesthesia if applicable. |
| Reverse narcotics or analgesics if applicable.a | Reverse narcotics or analgesics if applicable.a |
| Establish airway if possible or apply tight fitting mask and ventilate with 100% oxygen at 10-12 breaths per minute and 10 mm Hg airway pressure. | Establish airway if possible or use tight-fitting mask and ventilate with 100% oxygen at 10-12 breaths per minute and 10 mm Hg airway pressure. |
| Administer doxapram at 1-2 mg/kg IM, IV, IO. | Administer doxapram at 1-2 mg/kg IV, IO, IM. |
| If bradycardic, administer atropine at 0.02 mg/kg IV, IO (or glycopyrrolate in rabbits/rats at 0.01 mg/kg). | Begin chest compressions 100-120 per minute. |
| If successful, check blood pressure and correct fluid deficits. | Use vasopressin 0.8 U/kg IV, IO; double the dose if used via endotracheal tube. |
| Check temperature and correct if necessary. | If no response in 1 minute, consider epinephrine 0.01 mg/kg IV, IO, double the dose if used via endotracheal tube. |
| Begin diagnostic workup. | If ventricular fibrillation: defibrillate at 5-10 joules/kg x3. |
| Treat underlying disorders. | If unsuccessful, consider open-chest CPR. |
| If successful, check blood pressure and correct fluid deficits. | |
| Check temperature and correct if necessary. | |
| Begin diagnostic workup. | |
| Treat underlying disorders. |
a Atipamezole for medetomidine. Butorphanol or buprenorphine for mu-receptor analgesics (i.e., hydromorphone, morphine, fentanyl); these drugs are preferred to naloxone as they reverse mu-receptor respiratory and central depression, leaving kappa receptor analgesia effects intact; naloxone reverses both mu and kappa receptor and is very short acting.
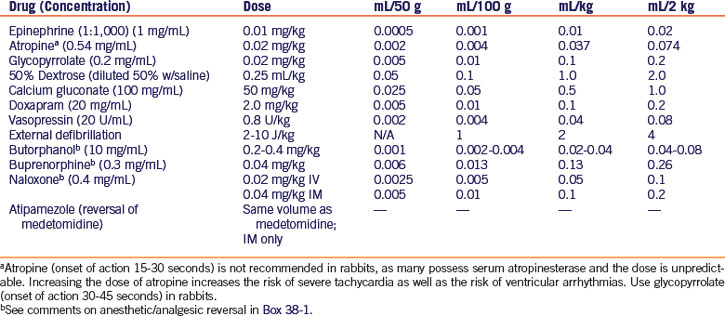
Epinephrine has routinely been the vasopressor of choice for ventricular fibrillation, asystole, and pulseless electrical activity (PEA), the condition of cardiac arrest with no mechanical activity of the heart but normal/slow electrical activity and ECG findings. However, epinephrine and other catecholamines lose much of their effectiveness as vasopressors when the body is in a state of hypoxia and acidosis. Vasopressin may be more effective under these conditions, and may improve rates of restoration of spontaneous circulation and survival.2,24 Vasopressin should be given first, followed by epinephrine. Both drugs may be given intravenously, intraosseously, or via the endotracheal tube. Dosages for CPCR drugs are given in Table 38-1.
Determining the Effectiveness of CPCR
The following parameters found useful in humans and traditional pet species may be useful in small mammals as well. The presence of palpable pulses is not an indication of adequate blood flow. Although palpable pulses may evaluate response to resuscitation, they do not indicate adequacy of organ perfusion. Two other measurements, end-tidal CO2 and blood gas measurement, can provide a more accurate assessment of organ perfusion.2 End-tidal CO2 should be measured during CPR, as a steady rise is more likely to be associated with a successful outcome. When end-tidal CO2 does not rise above 10 mm Hg after a resuscitation time of 15 to 20 minutes, the resuscitative effort is unlikely to be successful. End-tidal CO2 measurements are practical only in the intubated patient weighing more than 50 g.1,12
The common practice of monitoring arterial blood gases during CPR should be abandoned in favor of monitoring venous blood gases, as the latter represents the oxygenation and acid-base status of peripheral tissues.2
In traditional pet species, failure to regain consciousness in the first few hours after CPR does not necessarily indicate prolonged or permanent neurologic impairment. However, coma longer than 4 hours after CPR carries a poor prognosis for full neurologic recovery.2,24
Brainstem reflexes, most importantly the pupillary light reflex (PLR), can have prognostic value in patients that do not regain consciousness after CPCR. Absence of the pupillary light reflex after one or more days of coma indicates little to no chance of neurologic recovery. This reflex has no prognostic value in the first 6 hours after CPCR because it can be transiently lost and then reappear. It should be noted that atropine and epinephrine can produce pupillary dilation, but these do not interfere with the pupillary response to light.24
Anesthesia-Related Arrest
Anesthesia-related arrests represent one of the more treatable causes of arrest in veterinary patients. In the authors’ experience, exotic companion mammals under inhalant anesthesia often become bradycardic just prior to respiratory and cardiac arrest. Therefore use of an ultrasonic Doppler with concurrent measurement of blood pressure with or without ECG monitoring should be used during any anesthetic procedure, with measurements taken every 5 minutes.
In the event of respiratory arrest, discontinue any inhalant anesthesia, intubate the patient if this has not already been done, and initiate positive-pressure ventilation with 100% oxygen at a rate of 20 to 26 breaths per minute. If intubation is not possible, begin mask ventilation as described above. If other anesthetic/analgesic agents were given prior to anesthesia, administer reversal agents if applicable25 (see Table 38-1, Box 38-1). Doxapram is often useful as a respiratory stimulant.41
For cases of bradycardia, administer atropine intravenously, intraosseously, or via the endotracheal tube. Glycopyrrolate may be indicated in rabbits and rodents because of the presence of serum atropinase.29 Drug dosages are given in Table 38-1.
Shock and Fluid Therapy
Shock is defined as poor tissue perfusion from either low blood flow or unevenly distributed flow, resulting in an inadequate delivery of oxygen to the tissues.3 Hypovolemic shock is caused by absolute or relative inadequate blood volume. Absolute hypovolemia occurs with actual loss of blood—for example, arterial bleeding, gastrointestinal ulcers, or coagulopathies.3 With relative hypovolemia, there is no direct blood loss (hemorrhage) from the intravascular space. Examples include severe dehydration from gastrointestinal tract loss, significant loss of plasma (burns), or extensive loss of intravascular fluids into a body space such as the peritoneal cavity. In any case, there is decreased blood volume and venous return to the right side of the heart. This causes a reduction in return to the left side of the heart and consequently cardiac output.3 Studies in rabbits and rats show baroreceptor response to hypovolemia begins at 30% loss of blood volume. In other pet species, 30% loss also causes a decrease in blood pressure to below 60 mm Hg (diastolic) or less than 90 mm Hg (systolic). Carotid and aortic artery baroreceptors detect a decrease in stretch due to the decrease in cardiac output. This sends a neural signal to the vasomotor center in the medulla oblongata, resulting in inhibition of the vagal parasympathetic center and stimulation of the sympathetic center. The result is vasoconstriction of the veins and arterioles throughout the peripheral circulatory system and increases heart rate and strength of heart contraction. The humoral response to shock results in increased circulating catecholamines, which stimulates rennin release via adrenergic receptors on cells of the juxtaglomerular apparatus (specialized smooth muscle cells in the afferent arterioles). The release of renin stimulates activation of the renin-angiotensin-aldosterone system. These combined effects lead to a restoration of blood pressure, increased cardiac performance, and maximal venous return in the face of blood loss. Continued loss of blood volume results in hypovolemic shock and hypotension. Fluid therapy is required to optimize patient outcome.3
Three Phases of Hypovolemic Shock
Early or Compensatory Phase
The early or compensatory stage of shock occurs because of the baroreceptor-mediated release of catecholamines.3,34 Blood pressure increases because of the increase in cardiac output and systemic vascular resistance. This is the stage seen commonly in dogs with blood loss less than 20% of total blood volume. In the authors’ experience, exotic companion mammals rarely present in this stage of shock. Clinical signs in dogs include increased heart rate, normal or increased blood pressure, and normal or increased flow (bounding pulses and capillary refill in less than 1 second). The increased heart rate and normal or increased blood pressure is the key indicator of compensatory shock. Volume replacement at this stage is usually associated with a good outcome.
Early Decompensatory Phase
The second stage of shock occurs when fluid losses continue. There is a reduction in the blood flow to the kidneys, gastrointestinal tract, skin, and muscles. There is an uneven distribution of blood flow.3 This appears to be the most commonly encountered phase of shock in exotic companion mammals. Clinical signs include hypothermia, cool limbs and skin, tachycardia, normal or decreased blood pressure, pale mucous membranes, prolonged capillary refill time (CRT), and mental depression.3 Aggressive fluid therapy using crystalloids and colloids to support blood pressure and heart rate is required at this stage.
Decompensatory Phase
When a large volume of blood is lost, the neuroendocrine responses to hypovolemia become ineffective and irreversible organ failure begins. This is the final common pathway of all forms of shock in all species.3 Clinical signs are bradycardia with low cardiac output, severe hypotension, pale or cyanotic mucous membranes, absent capillary refill time, weak or absent pulses, hypothermia, oliguric to anuric renal failure, pulmonary edema, and a stuporous to comatose state. Cardiopulmonary arrest commonly occurs at this stage.3
Types of Fluids
Fluid choices include crystalloids and colloids. Individual characteristics of fluids influence the type and volume of fluid administered.14 Crystalloids include lactated Ringer’s solution, normal saline, and hypertonic saline (7.2%-7.5%). Hypertonic saline draws fluid into the intravascular space from all body compartments rapidly; therefore this can be extremely useful in selected cases.39 Natural colloids are blood, plasma, or albumin. Synthetic colloids include hetastarch (HES) (Hespan, Jorgensen Labs, Loveland, CO) and Oxyglobin (OPK Biotech, Cambridge, MA). Oxyglobin has the added advantage of carrying oxygen on the hemoglobin molecule to all the small vessels. Isotonic crystalloid solutions are commonly used together with colloids in the resuscitation phase.14
Warm all fluids to the body temperature of the patient regardless of the route of administration (see “Maintenance of Normothermia,” below). Fluids can be warmed to 100°F to 103°F (38°C-39°C) without affecting their composition.14 Dextrose solutions may be added to crystalloid solutions for the treatment of hypoglycemia confirmed via blood glucose measurement. Give an initial bolus of 50% dextrose at 0.25 mL/kg as a 1:1 dilution with isotonic saline intravenously; determine blood glucose 1 hour later. Administer additional dextrose as 1.25% with crystalloids, with frequent rechecks of blood glucose. Administer dextrose with care, as it may induce compartmental shifts in electrolytes and water, resulting in worsening hypovolemia.14
Fluid Resuscitation of the Critically Ill Small Mammal
Fluid therapy is used to correct life-threatening abnormalities in volume, electrolyte, and acid-base status. The goals of fluid therapy include resuscitation (correction of perfusion deficits), rehydration (correction of interstitial deficits), and maintenance.14
It is important to administer the least amount of fluids necessary to reach the desired endpoints of resuscitation. Clinical markers important to determine response to therapy and include restoration of normal mentation, mucous membrane color, CRT, and establishment of normothermia, normovolemia, and normal heart rate and urine output.14
Resuscitation implies an urgent need to restore tissue perfusion and oxygenation. The type, quantity, and rate of fluid administration required to reach the desired resuscitation endpoints are determined based on the phase of shock. These commonly include crystalloids with the addition of colloids.14 As mentioned earlier, exotic companion mammals typically present in the decompensatory phases of shock.
Earlier recommendations for shock therapy included crystalloids administered quickly in volumes equivalent to the patient’s blood volume. However, it should be kept in mind that resuscitation with crystalloids alone can result in a significant accumulation of pulmonary and pleural fluid. The resultant hypoxemia contributes to shock pathophysiology.14
The authors have found success with the following procedures for the treatment of hypovolemic shock in various exotic companion mammals (Box 38-2): administer a bolus infusion of 7.2% to 7.5% hypertonic saline (3 mL/kg IV or IO as a slow bolus over 10 minutes). The rapid effects of hypertonic saline are maintained with the addition of the colloid HES, which is given at 3 mL/kg IV or IO over 5 to 10 minutes. Indirect systolic blood pressure should be checked frequently (see “Measurement of Indirect Systolic Blood Pressure,” below). Once it is above 40 mm Hg systolic and/or there is improvement in other clinical markers of shock, administer maintenance isotonic crystalloids while aggressively rewarming the patient (see “Maintenance of Normothermia,” below). In many species, once rectal temperature approaches 98°F (36.7°C), adrenergic receptors begin to respond to catecholamines and fluid therapy.32 Temperatures during this rewarming phase must be checked frequently in all patients to prevent inadvertent hyperthermia. Blood pressure is rechecked when the temperature is greater than 98°F (36.7°C). Isotonic crystalloids (10 mL/kg) with HES in increments of 3 to 5 mL/kg can be repeated over 15 minutes until the systolic blood pressure rises above 90 mm Hg and/or there is again improvement in clinical markers. When the systolic blood pressure is above 90 mm Hg, the rehydration phase of fluid resuscitation begins. If the patient is known to be hypoproteinemic, administer a constant-rate infusion (CRI) of HES at 0.8 mL/kg per hour during rehydration, which will help maintain oncotic pressures in the intravascular space.
Box 38-2 Correction of Perfusion Deficits
Decompensatory phase of shock (bradycardia, hypotension, hypothermia):
Slow IV or IO bolus over 10 minutes of hypertonic saline 7.2%-7.5% (3 mL/kg) + hetastarch (3 mL/kg)
Begin external and core body temperature warming over 1-2 hours
Begin crystalloids at maintenance rate (3-4 mL/kg per hour)
Repeat boluses 3-4 times until blood pressure is normal:
1. Crystalloids (LRS, Normasol, Plasmalyte) at 10 mL/kg
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree