CHAPTER 51Embryo Transfer and Related Technologies
Embryo transfer (ET) in horses was first described in 19721–2 and has been performed commercially since the 1980s.3–6
ET has traditionally been most widely used in the older mare that fails to get pregnant or that conceives, establishes a pregnancy, and then undergoes early embryonic death (EED) or abortion. This limits the technology because these mares are less likely to provide embryos for transfer than normal, reproductively healthy mares.7,8 More recently ET has been used in performance mares (racing, showing, polo, cutting, etc.) and has been combined with newer technologies such as breeding with frozen semen, low dose insemination techniques, and other assisted reproductive techniques (ART) such as oocyte transfer and intracytoplasmic sperm injection (ICSI). Adoption of these techniques places pressure on researchers to develop methods of superovulation and better cryopreservation of larger embryos and even oocytes.
ET can be performed on yearling9 or 2-year-old mares as a method to get them into production 1 or 2 years earlier than traditional breeding. In addition, ET has been used to produce offspring from endangered equids such as the Przewalski’s horse, zebras,10 and the endangered Poitou donkey11 (Figure 51-1).
MANAGEMENT OF DONORS AND RECIPIENTS
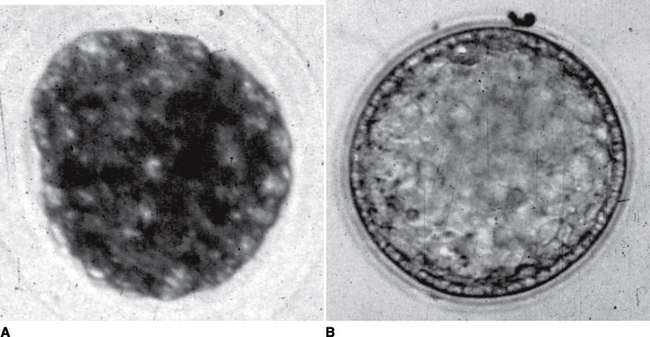
Embryos are usually collected on day 6, 7, or 8 (day 0 is day of ovulation) from naturally single or occasionally multiple ovulations. Embryos are collected 1 day later if the mare has been bred to frozen semen rather than fresh or cooled semen. There is an extremely rapid growth that occurs in the horse embryo from a morula to an expanding blastocyst (day 5 to day 7)12,13 that results in special characteristics defining embryo development (Figure 51-2).
Day 6 embryos are smaller, harder to harvest, and are the best developmental age for micromanipulation such as bisection14 or for freezing.15,16 Day 8 embryos are the largest size that can comfortably fit into a 0.25 ml pipette for transfer.
Factors that affect embryo recovery are age of the mare, semen quality, semen type (fresh, cooled, or frozen), and number of ovulations of the mare.4
Mares with a history of establishing pregnancies and then aborting are better candidates than those with a history of repeatedly returning to estrus after breeding, as they most likely will not provide a fertilized egg for recovery from the uterus. We have demonstrated a significantly lower recovery of embryos from mares with a history of infertility compared to normal experimental mares.4 This is not believed to be due to failure to identify the embryo or differences in fluid recovery. Apparently the poor recovery rates from infertile mares is due to absence of fertilization and/or altered gamete transport (which either prevents spermatozoa from reaching the egg or the fertilized egg from reaching the uterus) or an increased incidence of early embryonic death.8,17–20 Also, in our experience, multiple embryos per cycle are more frequently recovered from Thoroughbred and Warmblood mares compared to Quarter Horses or Arabian mares. In one study conducted over a total of 59 estrous cycles, a spontaneous multiple ovulation (>2 ovulations) was detected in 56% of cycles. The majority of ovulations occurred the same day (68.6%) or 1 day apart (27%). Embryo recovery 7 days after a single ovulation was 52.8% compared to 10.6% for spontaneously double ovulating mares (P < 0.001).21 Recovery rates for days 7, 8, and 9 post-ovulation were similar, but recovery of embryos on day 6 was slightly lower.22–25 The lower embryo recovery rate on day 6 may be attributed to (1) failure to identify the embryo in the recovery medium, (2) loss of the embryo during recovery procedure due to its small size, (3) failure to obtain the embryo in the uterine flush because of its greater specific gravity, or (4) failure of some embryos to enter the uterus by day 6. More than likely the latter 2 reasons are of greater significance. In one study involving 27 mares that failed to provide an embryo on day 6.5, 9 established a pregnancy without subsequently being rebred.26 Some of these pregnancies may have originated from a second ovulation that was not detected and occurred after cessation of palpation at the end of estrus. In another study, 143 embryo recovery attempts were performed on day 6. Ninety-two mares (64%) failed to provide an embryo. Eighty-three mares that failed to provide an embryo were subjected to embryo recovery again on day 7 postovulation. The recovery of 18 embryos on day 7 (22%), whose mean development stage (early blastocyst) and size (330 μm) were not significantly different from embryos recovered on day 6, suggested that failure of recovery on day 6 was due to delayed oviductal transport, not flushing technique.26 Embryo recovery 6.5 days after ovulation in superovulated mares was 1.5 versus 2.6 when these same mares were flushed 7.5 days after ovulation.27
Embryo Recovery
Methods of recovery have not varied much from the original description of Imel.3 A catheter is introduced into the vagina through the cervix and approximately 5 cm into the uterine body. Once in position, the cuff of the catheter is inflated with 60 ml of sterile saline or air. The catheter is then drawn back against the internal os of the cervix to assure a tight seal. A buffered solution (e.g., Dulbeccos phosphate buffered saline) is infused into the uterus (1 to 2 liters at a time) and recovered by gravity through a 75 micron filter. The procedure is repeated at least 3 times but commonly more often (up to 6). A study of how to improve embryo recovery demonstrated that embryos were recovered on the first 3 liters in 31.6% of attempts from 209 client mares. A total of 32 additional embryos were subsequently recovered during the extra flush attempt with 2 additional liters of medium.28 If the media is clear it may be reused immediately on the same mare. The filtered media is tipped into a search dish and is then examined under 7 to 15× magnification with a stereo dissection microscope.
Occasionally it may be necessary to perform the flush in a mare with her cervix fully dilated and a hand inserted in the uterus. In these cases fluids are difficult to contain within the uterus. It has been suggested that embryo recovery rates will be improved by allowing some time (3 minutes) from infusion of the first flushing solution until recovery29 and also after administering oxytocin.
Recipient Mares
Proper selection and management of recipient mares is probably the most important factor affecting success of an equine embryo transfer program. Maiden mares or young mares that have a history of producing 1 or 2 foals are preferred as recipients. Clients have a right to expect quiet and well-handled mares as recipients. We prefer recipient mares that weigh 450 to 550 kg, and are 3 to 10 years of age and halter broken. The method of matching recipients with donors in our embryo transfer program may be unique because of the large number of recipient mares we maintain. Usually a donor can be matched with a recipient that has ovulated spontaneously, without using hormones to synchronize their cycle. Where large numbers of recipient mares are not available, synchronization of ovulation can be provided by hormonal therapy (see Chapter 3). It is preferable that at least 2 recipients are available for each donor mare. A full reproductive exam is performed on all mares. Recipient mares should be fed to maintain good body weight and condition. An effort is made to use only those recipients that have experienced 2 or more normal cycles. Recipients are selected that have ovulated 1 day prior (+1) or 0 to 3 days after (0, −1, −2, −3) the donor mare.
Mares that receive an embryo nonsurgically are treated with antibiotics and Regumate for 5 days after transfer. Recipients may be examined with ultrasonography at 11 to 12 days of gestation (4 to 5 days post-transfer).26,30,31 Mares are reexamined on days 15, 25, 35, 45, and 60 of pregnancy. Embryonic death between day 15 and 60 of gestation appears to be no greater in embryo transfer recipients than in mares inseminated with fresh semen.32 The influence of the size of the recipient on the ease of foaling and the subsequent size of the foal is an unresolved issue. It certainly is important from the client’s perspective that he or she receive a recipient that is big enough to let the foal be born a normal size. The experiments reported of Walton and Hammond33 and of Tischner34 are indicative of our philosophy, in that the size and health of the uterus determine the size of the foal at term. This was also supported by work from Newmarket:
The influence of maternal size on foal birthweights and the total microscopic area of fetomaternal contact at the placental interface was studied by comparing placentae from normal Thoroughbred-in-Thoroughbred and Pony-in-Pony control pregnancies with those from between-breed Thoroughbred-in-Pony (deprived in utero environment) and Pony-in-Thoroughbred (luxurious in utero environment) pregnancies created by embryo transfer. Strong positive correlations were revealed between foal birthweight and both the gross area of the placenta and the total microscopic area of fetomaternal contact via the microcotyledons. The results highlight the importance of the intrauterine environment for the growth and development of the equine fetal foal.35
A development in the late 1980s in equine embryo transfer was the use of ovariectomized steroid-treated mares as recipients.36–38 It is necessary before transfer of the embryo to treat the ovariectomized mare with progesterone or progestins (synthetic progesterone-like compounds) for 4 to 7 days before transfer until between day 100 and day 140 of pregnancy (when placental progestogens will maintain pregnancy).39 Pregnancy rates after surgical transfer into ovariectomized progestin treated mares were similar to intact controls (70%).38 Parturition and lactation of ovariectomized embryo transfer recipients have been reported to be normal.40 The use of ovariectomized steroid-treated mares as recipients eliminates the need for synchrony of ovulation between donor and recipient and reduces the number of recipients per donor; however, the need for daily administration of progestins reduces its appeal dramatically. To further demonstrate that the uterus and progesterone were all that were necessary to nourish an equine pregnancy, it was reported that foals were born after ET into mares with gonadal dysgenesis.41 Early in the breeding season, when mares are not cycling naturally, intact mares can be used as recipients by supplementation with a progestin.
Embryo Transfer
In earlier publications on ET we advocated the use of surgical transfer of equine embryos4 as various studies on nonsurgical transfer of equine embryos have been attempted by others7,42–44 with extremely variable results (12.5%-71% pregnancy rates). Currently we restrict the use of surgical transfer to ART (such as ICSI, GIFT, or oocyte transfer) because pregnancy rates have been greater than 75% per embryo transferred at day 15 for more than 8 years using nonsurgical transfer. Many variations of nonsurgical transfer have been reported45,46; however, careful management of delicate tissues in standard nonsurgical ET will result in consistent pregnancy rates without the need to grasp novel procedures. Our equipment preference is the Cassou (IMV, L’Aigle, France) ET transfer pipette (0.25 ml straw), which has a sterile disposable outer sheath and a thin plastic wrap (sanitary sleeve) that the transfer pipette pushes through as the cervix is penetrated.
Factors Affecting Pregnancy Rates
The majority of embryo transfers have been performed 6 to 8 days post-ovulation. Limited studies are available addressing the effect of embryo age on pregnancy rate. From results of experiments22,23,25,47 it appears that the older, larger embryos are less viable in nonsurgical transfer and possibly even surgical transfer. The older embryos (>8 days), with their increased fluid-volume-to-surface ratio, may not withstand the shock of recovery and transfer to recipients as well as smaller embryos. Despite this, we have produced foals transferring embryos at 10 days of age (Figure 51-3). Once we identify the mare is pregnant using ultrasonography, we then harvest and transfer the embryo. No expensive identification equipment is necessary; however, the catheter used to flush the mare is necessarily large. The first foal from this technique was born at the Goulburn Valley Equine Hospital (GVEH) in 1993. To date the 10-day nonsurgical transferred pregnancy rates are not very encouraging.
As the morphology of the embryo becomes more abnormal, the possibility of a pregnancy resulting from transfer of the embryo decreases.4 Also interesting is the reported lower pregnancy rates and higher embryonic loss after ET from older mares.16
On Day 7 or 8 after ovulation, embryos (fresh or cooled/transported) were transferred by surgical or nonsurgical techniques into recipients ovulating from 5 to 9 d before transfer. At 12 and 50 d of gestation (Day 0 = day of ovulation), pregnancy rates were 65.7% (419 of 638) and 55.5% (354 of 638). Pregnancy rates on Day 50 were significantly higher for recipients that had excellent to good uterine tone or were graded as “acceptable” during a pretransfer examination, usually performed 5 d after ovulation, versus recipients that had fair to poor uterine tone or were graded “marginally acceptable.” Embryonic factors that significantly affected pregnancy rates were morphology grade, diameter and stage of development. The incidence of early embryonic death was 15.5% (65 of 419) from Days 12 to 50. Embryo loss rates were significantly higher in recipients used 7 or 9 d vs 5 or 6 d after ovulation. Embryos with minor morphological changes (Grade 2) resulted in more ( P < 0.05) embryo death than embryos with no morphological abnormalities (Grade 1). Between Days 12 and 50, the highest incidence of embryo death occurred during the interval from Days 17 to 25 of gestation. Embryonic vesicles that were imaged with ultrasound during the first pregnancy exam (5 d after transfer) resulted in significantly fewer embryonic deaths than vesicles not imaged until subsequent exams. In the present study, embryo morphology was predictive of the potential for an embryo to result in a viable pregnancy. Delayed development of the embryo upon collection from the donor or delayed development of the embryonic vesicle within the recipient’s uterus was associated with a higher incidence of pregnancy failure. Recipient selection (age, day after ovulation, quality on Day 5) significantly affected pregnancy and embryo loss rates.48
Embryo Storage
Cooled, transported embryos increase the flexibility of equine ET by allowing major centers to maintain large numbers of recipients for transfer of embryos collected from remote locations.49 This means that experienced personnel can perform the transfer and that the expenses of maintaining a herd for a small population of donor mares are eliminated. The procedures for cooling and transporting equine embryos were first described in 1987.50 Originally the transport media described was Ham’s F10 with a 5% CO2gas mixture to buffer it.50 The main drawback to this media was the inability to maintain the media in a state ready for use. Preparation of media for cooling or transport of embryos under field conditions would be more practical with the use of a nongassed medium such as Ham’s F10 with Hepes buffer. However, day 14 pregnancy rates for embryos transferred surgically after storage in Ham’s F10 plus 10% fetal calf serum plus 5% CO2, 5% O2, and 90% N2for 24 hours at 5°C were better (P < 0.05) (14/20, 70%) than embryos maintained identically but with Hepes buffering of the Ham’s F10 media (4/20, 20%).51 Results from more recent work suggest that other media (EmCare, ICP, Auckland, New Zealand, or Vigro Holding Plus, A-B Technology, Pullman, WA) are also useable.52–55 These aforementioned commercial media do not have to be gassed and come in small, disposable vials, making them attractive for commercial use.
Pregnancy rates from embryos cooled and transported are similar to transfer of embryos immediately.56
Frozen embryo technology has lagged behind other equine ET developments. This may be due in part to a perceived lack of applicability due to the inability to repeatedly harvest multiple embryos and in part to breed registry restrictions on transferring frozen embryos. Technical difficulties with freezing larger embryos may also be a contributing factor. Advantages of freezing embryos include (1) embryos can be stored indefinitely, thus preserving important genetic lines; (2) embryos can be collected from donors and transferred into recipients at a later time, thus minimizing the number of recipients; and (3) embryos can be transported within and exported from countries at our convenience without regard to matching of recipient cycles. Cryopreservation of equine embryos15,57,58 resulted in reasonable pregnancy rates. Results can be improved further if only embryos at the morula or early blastocyst stage of development are frozen. An interesting development that nicely demonstrates the flexibility of ET is the freezing of equine embryos in the breeding season and successful establishment of pregnancies after transfer in the nonbreeding season into ovariectomized mares treated for 6 days with Regumate before transfer.59 In these cases, recipients need to be kept on Regumate treatment until at least 100 days of pregnancy.60
Techniques involve addition of glycerol in 1 or 2 steps to a final concentration of 10%. Other cryoprotectants such as 1,2-propanediol and ethylene glycol have been examined.61,62 Numerous studies have been published with the following general conclusions consistently reported.59,61–64 Success from cryopreservation of larger embryos is still not commercially viable. The protocol first reported by Slade et al15 is quite efficacious for embryos less than 200 μm and can be modified to remove glycerol in 3 steps (inclusive of 10% sucrose).
Expected pregnancy rates from smaller embryos (late morulas and early blastocysts, i.e., <300 microns) after freeze-thaw is around 70%.15,65,66 Success with larger, expanded blastocysts (>300 microns) is much less, at around 10% to 20%.15,58,59 One study reported good pregnancy rates from larger embryos at 57%.66 The exact reason for failure of large embryos to survive the freeze-thaw process is not currently known, although it possibly relates to differences in permeability to cryoprotectants at different embryo ages,61 which is probably related to the capsule64,67 and the recognition that the inner cell mass cells are more susceptible to damage during cryopreservation than the trophectoderm.68
Recent research on equine embryo preservation has concentrated on vitrification of embryos.69 Vitrification is explained well by the following quotation.
The failure of complex mammalian organs, such as the kidney, to function following freezing to low temperatures is thought to be due largely to mechanical disruption of the intercellular architecture by the formation of extracellular ice. Classical approaches to the avoidance of ice formation through the imposition of ultra-rapid cooling and warming rates or by gradual depression of the equilibrium freezing point during cooling to −80 degrees C have not been adequate. An alternative approach relies on the ability of highly concentrated aqueous solutions of cryoprotective agents to supercool to very low temperatures. At sufficiently low temperatures, these solutions become so viscous that they solidify without the formation of ice, a process termed vitrification. When embryo suspensions are cryopreserved using conventional procedures, this supercooling behaviour allows intracellular vitrification, even in the presence of extracellular ice. We have therefore used mouse embryos to examine the feasibility of obtaining high survival following vitrification of both the intra- and extracellular solutions and report here that in properly controlled conditions embryos seem to survive in high proportions after cryopreservation in the absence of ice.70
Theoretically, vitrification will result in more time efficiency and does not involve expensive computer-controlled freezing rate equipment.69 Initial studies with vitrification were quite promising, with demonstration of similar pregnancy rates from open pulled straw systems and a cryoloop versus conventional techniques:
The open pulled straw (OPS) and cryoloop have been used for very rapid cooling and warming rates. The objective of this experiment was to compare efficacy of vitrification of embryos in OPS and the cryoloop to conventional slow cool procedures using 0.25 mL straws. Grade 1 or 2 morulae and early blastocysts (< or = 300 microm in diameter) were recovered from mares on Day 6 or 7 post ovulation. Twenty-seven embryos were assigned to three cryopreservation treatments: (1) conventional slow cooling (0.5 degrees C/min) with 1.8 M ethylene glycol (EG) and 0.1 M sucrose, (4) vitrification in OPS in 16.5% EG, 16.5% DMSO and 0.5 M sucrose, or (3) vitrification with a cryoloop in 17.5% EG, 17.5% DMSO, 1 M sucrose and 0.25 microM ficoll. Embryos were evaluated for size and morphological quality (Grade 1 to 4) before freezing, after thawing, and after culture for 20 h. In addition, propidium iodide (PI) and Hoechst 33342 staining were used to assess percent live cells after culture. There were no differences ( P > 0.1) in morphological grade or percent live cells among methods. Mean grades for embryos after culture were 2.9 +/− 0.2, 3.1 +/− 0.1, and 3.3 +/− 0.2 for conventional slow cooling, OPS and cryoloop methods, respectively. Embryo grade and percent live cells were correlated, r = 0.66 ( P < 0.004). Thus OPS and the cryoloop were similarly effective to conventional slow-cooling procedures for cryopreserving small equine embryos.71
This and other studies have utilized embryo culture and laboratory techniques to assess embryo viability.71–73
In a preliminary study (Experiment 1), embryos were exposed in three steps to vitrification solutions containing increasing concentrations of ethylene glycol and glycerol (EG/G); the final vitrification solution was 3.4 M glycerol + 4.6 M ethylene glycol in a base medium of phosphate-buffered saline. Embryos were warmed in a two-step dilution and transferred into uteri of recipients. No pregnancies were observed after transfer of blastocysts >300 μm (n = 3). Transfer of morulae or blastocysts <= 300 μm resulted in four embryonic vesicles (4/6, 67%). In a second experiment, embryo recovery per ovulation was similar for collections on Day 6 (28/36, 78%) versus Days 7 and 8 (30/48, 62%). Embryos <= 300 and >300 μm were vitrified, thawed and transferred as in Experiment 1. Some embryos <300 μm were also transferred using a direct-transfer procedure (DT). Embryo development rates to Day 16 were not different for embryos <= 300 μm that were treated as in Experiment 1 (10/22, 46%) or transferred by DT (16/26, 62%). Embryos >300 μm (n = 19) did not produce embryonic vesicles.69
Recently, 20 embryos were transferred that had been cooled for 12 to 19 hours, then vitrified. Thirteen of 20 (65%) mares became pregnant compared to 15 of 20 pregnancies from embryos vitrified immediately after collection.28 Clearly this study has supported the potential of on-farm embryo collection followed by transport to a facility capable of handling vitrification.
Superovulation
Some of the advantages of superovulation would be (1) the possibility for collection of multiple embryos, (2) higher pregnancy rates per embryo recovery attempt, (3) an increase in the number of embryos available to freeze, and (4) an increase in the number of preovulatory follicles that may enable harvesting of more in vivo matured oocytes. Other benefits not strictly related to ET would be improving pregnancy rate from problem breeders and better pregnancy rates from subfertile stallions74 or from frozen semen from some stallions. Even an increase in the number of pregnancies per cycle would be a huge boon to the breeding industry, providing sophisticated techniques of twin or multiple vesicle elimination where available.74 Some of the problems are (1) inconsistency in number of mares responding to superovulation treatments, (2) lack of availability of commercial superovulatory drugs, (3) considerable cost of the superovulation treatment, and (4) possible lowered viability of multiple embryos.
Gonadotropin-Releasing Hormone (GnRH)
GnRH has been reported to induce ovulation in seasonally anestrus mares75–77; however it has been demonstrated to be ineffective in cycling mares during the regular breeding season for induction of multiple ovulation.78,79
Equine Pituitary Extract (EPE)
EPE is a crude pituitary extract obtained from extraction80 of abattoir-derived pituitaries containing 6% to 10% LH and 2% to 4% FSH. It has been clearly demonstrated that multiple ovulation can be induced in both seasonally anovulatory and cycling mares with EPE.81–85 However, the induction of ovulation in the seasonally anovulatory mare is impractical because a large number of those mares fail to continue to cycle after the induced ovulation and if they become pregnant fail to maintain the corpus luteum (CL). In addition, effective doses of pituitary extract for induction of multiple ovulation are higher in anovulatory mares. Pituitary extract administered during diestrus for a period of approximately 1 week appears to be an effective means of inducing multiple ovulation in cycling mares. However, ovulation rates are quite low in comparison to those obtained in cattle, usually ranging from 2 to 4 ovulations per mare. A more refined form of EPE has just become available (see FSH below). There are two questions, however, that have not been adequately resolved. Are multiple ovulations associated with a reduced number of embryos per ovulation? Are they reduced in viability? It is most likely that the reduced number of embryos (embryos/ovulation) is related to those mares that have multiple ovulations on the same ovary. In relation to viability, although it was suggested that reduction in viability of multiple embryos occurred by one group,84,85 much of this was recorded before ultrasonography. Subsequent experiments21 refuted earlier work and encouraged the use of embryos collected from superovulatory programs. One study86 was able to show that fertilization rates and embryonic development from oviductal embryos collected from either EPE-treated mares or controls were identical. Purification of EPE by removing luteinizing hormone (LH) has not resulted in an improvement in ovulations or embryo recovery rate.87,88 A pregnancy rate of 70% was reported for embryos collected from superovulated mares after vitrification and transfer.28 The superovulation response was increased by increasing the frequency of administration,89 and this was subsequently shown to be associated with the total dose of EPE: