Chapter 15
Effusions
Abdominal, Thoracic, and Pericardial
Thoracic and Abdominal Effusions
Dogs and cats with thoracic effusions often show dyspnea as the most common clinical sign.1,2 Other clinical signs include a crouched, sternal recumbent position with extension of the head and neck; open-mouth breathing; tachypnea; and forceful abdominal respiration. Cyanosis may be present. With milder effusions, lethargy and lack of stamina may be the only clinical signs. Animals, especially cats, with mild to moderately severe effusions often adapt by decreasing their activity, thus concealing their illness until it is severe. With chronic pleural effusion, dogs and cats may present with coughing as the only clinical sign.1 Physical findings with pleural effusion depend on the amount of fluid present but include muffled heart and lung sounds.
Pericardial Effusions
Pericardial effusions in the cat are often secondary to congestive heart failure or feline infectious peritonitis but may be caused by primary cardiac neoplasia such as lymphoma.3 The most common causes of pericardial effusion in the dog include cardiac neoplasia and idiopathic pericardial effusion.4,5 Hemangiosarcoma is the most common cardiac neoplasm reported, but other reported neoplasms include chemodectoma, lymphoma, and thyroid carcinoma.4 Other less common causes include cardiac disease, inflammatory or infectious diseases, trauma, coagulopathy, and congenital defects.
Collection Techniques
Thoracocentesis
Pleural effusions are typically abundant and bilateral but may be mild, unilateral, compartmentalized, or both. Radiography and ultrasonography help determine the extent and location of the effusion and guide thoracocentesis. If the fluid is not compartmentalized, thoracocentesis is performed approximately two thirds down the chest, near the costochondral junction at the sixth, seventh, or eighth intercostal space.
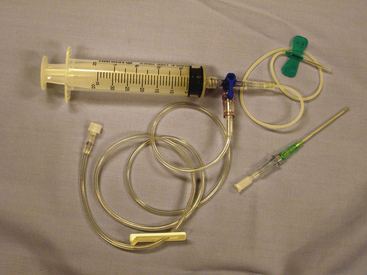
The needle should be inserted next to the cranial surface of the rib to minimize the risk of lacerating the vessels on the rib’s caudal border. As long as the needle or catheter is below the fluid line, air will not be aspirated into the thoracic cavity. If only a single syringe of fluid is to be collected, the syringe may be attached to the catheter, the fluid aspirated, and the catheter withdrawn with the syringe attached. If a larger volume of fluid is to be removed or the syringe is to be repeatedly filled, extension tubing and a three-way stopcock should be attached (Figure 15-1).
Abdominocentesis
The ventral midline of the abdomen, 1 to 2 centimeters (cm) caudal to the umbilicus, is the usual site of needle insertion. This site avoids the falciform fat, which may block the needle barrel. The urinary bladder is emptied to help avoid accidental cystocentesis. The site of needle insertion is shaved and aseptically prepared. Neither local nor general anesthesia is usually needed. With the animal in lateral recumbency, a ventral midline puncture is made using a 1- to 1½-inch, 20- to 22-gauge needle, or a 16- to 20-gauge, 1½- to 2-inch plain or fenestrated over-the-needle catheter without the syringe attached. Free-flowing fluid should be collected into appropriate collection tubes. The needle may be rotated if fluid is not visible in the needle hub or a syringe may be attached and gentle negative pressure applied.6 If a previous surgical incision is present, the needle should be inserted at least 1.5 cm away from the site to avoid abdominal viscera that may have adhered to the abdominal wall in the area of the scar. To enhance fluid collection, abdominal compression may be applied when an over-the-needle catheter is used after the stylet has been removed, leaving only the catheter in the abdominal cavity. Although the catheter may kink, sufficient fluid can usually be collected for analysis.
If the technique described above fails to yield fluid, four-quadrant paracentesis or diagnostic peritoneal lavage (DPL) may be performed. In four-quadrant paracentesis, the umbilicus serves as a central point, and centesis, as previously described, is performed in the right and left cranial and caudal quadrants.7 If DPL is performed, the animal is placed in dorsal recumbency, the area clipped and aseptically prepared, and a small 2-cm incision caudal to the umbilicus is made. A peritoneal lavage catheter without the trocar is inserted into the abdominal cavity and directed caudally into the pelvis. A syringe is attached and gentle suction applied. If no fluid is obtained, 20 mL/kg of warm sterile saline may be infused into the abdominal cavity. The patient is then rolled from side to side and the fluid collected via gravity drainage.6,7
Pericardiocentesis
Pericardiocentesis may be performed with the animal in the standing position or in sternal or left lateral recumbency. Adequate restraint is needed to avoid cardiac puncture, coronary artery laceration, or pulmonary laceration. Sedation is used as necessary. Electrocardiogram (ECG) monitoring is recommended but not essential. Cardiac contact with the catheter or needle usually causes an arrhythmia. A large area of the right hemithorax from the third to the eighth rib is shaved and aseptically prepared. Local anesthesia, including infiltration of the pleura with lidocaine, may be used to minimize discomfort associated with pleura penetration. Puncture is generally made between the fourth and fifth intercostal spaces at the costochondral junction. The needle is attached to a three-way stopcock, extension tubing, and syringe, and gentle negative pressure is applied.5,8
Slide Preparation and Staining
Clear, colorless fluids are usually transudates (low protein content and low cellularity). Preparation of sedimented or cytospin concentrated slides from low cellularity fluids aid in cytologic evaluation. Clear-amber and mildly opaque fluids are often modified transudates of low to moderate cellularity. Moderately to markedly opaque fluids, however, are usually exudates of moderate to very high cellularity. Slide preparation and staining techniques for various types of fluids are additionally presented in Chapter 1.
Sediment smears should be made on all nonturbid fluid specimens. This is done by centrifuging the fluid for 5 minutes at 165 to 360 gravity (G). This may be achieved in a centrifuge with a radial arm length of 14.6 cm by centrifuging the fluid at 1000 to 1500 revolutions per minute (rpm). After centrifugation, nearly all of the supernatant is poured off, leaving only about 0.5 mL of fluid with the pellet in the bottom of the tube. The supernatant may be used for total protein and chemical analysis (avoid EDTA samples which interfere with chemical analyses). The pellet is then resuspended in the remaining 0.5 mL of fluid by gentle agitation, a drop of the suspension is placed on a glass slide, and a routine pull smear or squash prep is made (see Chapter 1). The smear is air-dried and then stained with an appropriate hematologic stain.
Laboratory Data
Cell Counts and Counting Techniques
Accurate nucleated cell counts may be determined at commercial laboratories on many modern automated cell counters or by manual methods using a hemacytometer, however, cell clumping, cell fragmentation, and noncellular debris may cause counting errors with automated and manual techniques.10 Determination of nucleated cell counts should be performed on EDTA-preserved fluid to prevent clot formation or clumping of the sample. Serum separator tubes may introduce artifact to the count and therefore should not be used. A nucleated cell differential may be made on cytology preparations and often aids in classification of the effusion type.
Automated red blood cell (RBC) counts may help determine the amount of blood in the effusion. If grossly bloody fluid is obtained, a packed cell volume (PCV) may be performed. The RBC count, together with cytologic assessment (identification of platelets, erythrophagocytosis, intracellular and extracellular heme pigments such as hemosiderin and hematoidin), may aid in determination of the origin of the blood as far as iatrogenic blood contamination, per-acute hemorrhage, chronic hemorrhage, and increased capillary permeability with diapedesis of RBC into the cavity.10
Total Protein Measurement and Techniques
Fluid total protein concentration is used with the nucleated cell count to classify effusions as transudates, modified transudates, or exudates and to estimate the severity of inflammation, if present. The total protein content may be determined biochemically or estimated by refractometry. For ease and accuracy, the method of choice for determining total protein concentrations in effusions is refractometry. If the fluid is opaque, it is best to determine the refractive index of the supernatant after centrifugation, as the refraction of light by suspended nonprotein particles (i.e., lipoproteins, urea, cholesterol, and glucose) may result in an erroneous total protein reading.10,11 It must be kept in mind that chylous or lipemic fluids may not separate sufficiently to allow total protein to be estimated by refractometry or chemical methods.
Biochemical Analysis
In conjunction with fluid analysis, measurement of abdominal effusion supernatant bilirubin and creatinine and comparison with serum values may be performed to diagnose bile peritonitis and uroperitoneum, respectively. Additionally, when a white, opaque effusion is obtained, measurement and comparison of effusion supernatant and serum triglyceride and cholesterol values may be used to distinguish between pseudochylous and chylous effusions (Table 15-1). Specific biochemical analyses performed on effusions will be highlighted in the section titled “Specific Disorders Causing Effusions.”
TABLE 15-1
Ancillary Tests: Biochemical Analysis of Effusion Fluid
| Biochemical Test | Indications | Interpretation |
| Bilirubin | Bile peritonitis | Two-fold or greater concentration in effusion fluid than serum supports bile peritonitis. |
| Creatinine | Uroperitoneum | Two-fold or greater concentration in effusion fluid versus serum supports uroperitoneum. |
| Triglyceride | Chylous effusion | Triglyceride level greater than 100 milligrams per deciliter (mg/dL) in fluid supports chylous effusion. |
| Cholesterol | Nonchylous effusion | Higher concentration of cholesterol in effusion fluid versus serum supports nonchylous effusion. |
Microbiologic Cultures
Effusion fluid may be submitted for either bacterial or fungal cultures. It is best to contact the laboratory for specific information regarding submission protocol, types of transport containers and media, as many laboratories will provide special transport tubes and, in the case of anaerobic bacterial culture, special anaerobic tubes or media. In general, if the cytologic evaluation of an effusion suggests bacterial infection caused by the presence of large numbers of neutrophils, or if definitive bacteria are identified, the fluid should be cultured for both aerobic and anaerobic bacteria. Fluid samples for aerobic and anaerobic cultures should be collected using aseptic technique to avoid contamination. Fluid should be placed into a sterile tube (i.e., sterile red top tube) without EDTA, which may be bacteriostatic or bactericidal. Note that some transport systems support both aerobic and anaerobic bacteria. Submission of fluid for fungal culture should be performed as in the case of fluid submitted for aerobic bacterial culture. Chapter 1 contains a detailed discussion of methods for collection and transportation of samples for microbiologic culture.
Cells and Structures Seen in Effusions
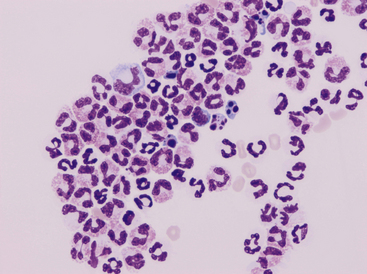
Neutrophils
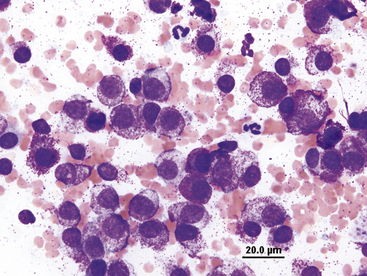
Neutrophils are present to some degree in most effusions and tend to predominate in effusions associated with inflammation. Cytologically, two general classes of neutrophils exist: (1) degenerate and (2) nondegenerate. Degenerate neutrophils are neutrophils that have undergone hydropic degeneration. This is a morphologic change that occurs in tissue and effusion neutrophils secondary to bacterial toxins that alter cell membrane permeability. The toxins allow water to diffuse into the cell and through the nuclear pores, causing the nucleus to swell, fill more of the cytoplasm, and stain homogeneously eosinophilic. This swollen, loose, homogeneous, eosinophilic nuclear chromatin pattern characterizes the degenerate neutrophil (Figure 15-2). Although all cell types are exposed to the same toxin, degenerative change is evaluated only in neutrophils.
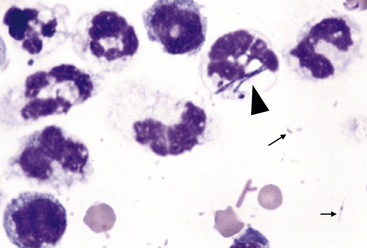
Figure 15-2 Numerous degenerate neutrophils with swollen nuclear chromatin.
Phagocytized bacterial rods (arrowhead) and extracellular bacteria are in the background (arrows).
Nondegenerate neutrophils such as peripheral blood neutrophils are those with tightly clumped, basophilic nuclear chromatin (Figure 15-3). Some neutrophils in effusions may be hypersegmented. Hypersegmentation is an age-related change; the nuclear chromatin condenses and eventually breaks into round, tightly clumped spheres (pyknosis) (Figure 15-4). These aged neutrophils are often seen phagocytized by macrophages (cytophagia) (Figure 15-5). The presence of nondegenerate neutrophils suggests that the fluid is not septic; however, bacteria that are not strong toxin producers, for example, Actinomyces spp., may be associated with nondegenerate neutrophils. Also, some infectious agents such as Ehrlichia and Toxoplasma spp. and various fungi may be associated with nondegenerate neutrophils.
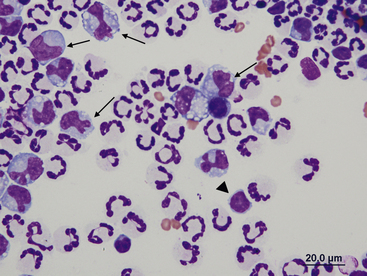
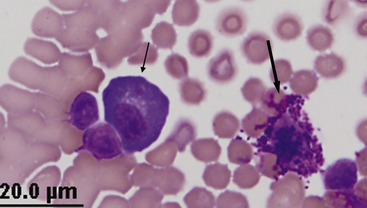
Figure 15-3 Feline abdominal fluid.
Numerous nondegenerate neutrophils. The chromatin is clumped and segmented. Also present are macrophages (arrows) and a small lymphocyte (arrowhead).
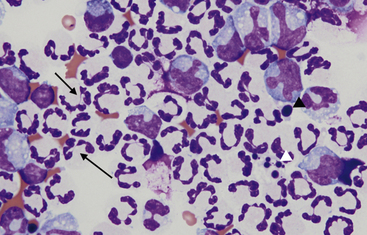
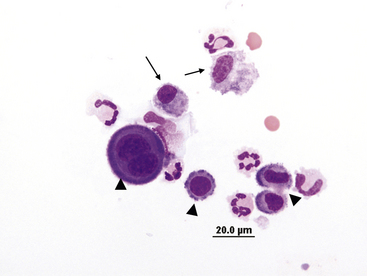
Figure 15-4 Feline abdominal fluid.
Numerous nondegenerate neutrophils are present; some are hypersegmented with only thin chromatin strands connecting the segments (small arrows). Pyknotic nuclei (arrowheads) are also present.
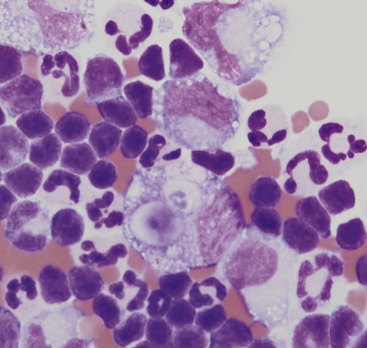
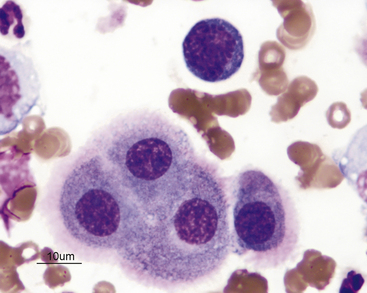
Figure 15-5 Abdominal fluid from a cat.
A large macrophage is present in the center, with a phagocytized remnant of a neutrophil. Numerous small lymphocytes and macrophages are present.
Effusions may also contain toxic neutrophils. Toxic changes (i.e., Döhle bodies, toxic granulation, diffuse cytoplasmic basophilia, foamy cytoplasm) develop in the bone marrow in response to accelerated granulopoiesis caused by inflammation. Toxic neutrophils in the peripheral blood migrate into the body cavity and are observed in effusions of the cavity.10 Although foamy cytoplasm is considered a toxic change, cytoplasmic vacuolation may be seen in neutrophils of peritoneal or thoracic fluid smears because of age-related change or EDTA-induced artifact.
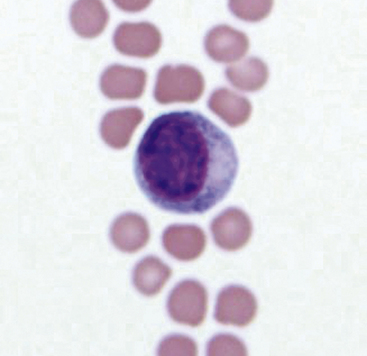
Mesothelial Cells
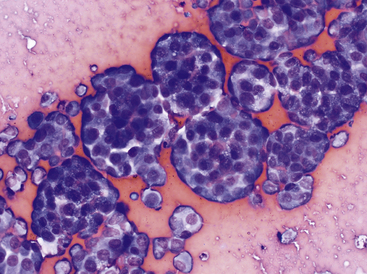
Mesothelial cells line pleural, peritoneal, and pericardial cavities as well as visceral surfaces, and are present in variable numbers in most effusions. Mesothelium easily becomes activated and reactive or hyperplastic in the face of inflammation or fluid accumulation of any type. In effusions, mesothelial cells are large, round cells present singly or in clusters, with singular, round central nuclei. Multinucleation occurs when cells are reactive (Figure 15-6). The nuclear chromatin has a fine reticular pattern and nucleoli may be present. Normal mesothelial cells are often noted to have moderate amounts of pale blue cytoplasm, and a pronounced pink cytoplasmic coronal fringe is often present in reactive cells (Figure 15-7). Mesothelial cells may be present in low or moderate numbers, as individual cells, in small clusters, and sometimes in large, three-dimensional balls (Figure 15-8). Reactive mesothelial cells may have several morphologic characteristics of malignancy and may be easily confused with neoplastic cells.
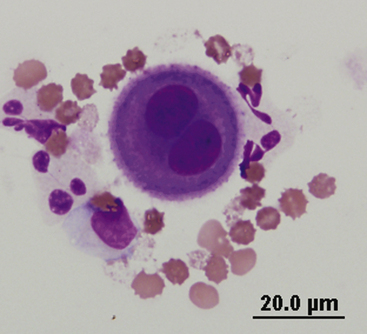
Figure 15-6 A binucleate mesothelial cell with deeply basophilic cytoplasm and an encircling eosinophilic fringe border.
Macrophages
Macrophages found in effusions generally have a single oval to bean-shaped nucleus and resemble tissue macrophages. The nuclear chromatin is lacy and cytoplasm is frequently vacuolated and may contain phagocytized debris or degenerate cells (Figure 15-9 and Figure 15-10).
Lymphocytes
Lymphocytes are commonly seen in effusions and are often the predominant cell type in chylous effusions (Figure 15-11) and neoplastic effusions secondary to lymphoid malignancy (Figure 15-12 and Figure 15-13). Small to intermediate or medium-sized lymphocytes present in body cavity fluids will appear similar to peripheral lymphocytes, with a scant rim of blue cytoplasm, round nucleus with evenly clumped to coarse chromatin, and no apparent nucleoli. Normal fluids will have low numbers of lymphocytes. Reactive lymphocytes may be seen in inflammatory effusions and are slightly larger than small lymphocytes, with deeply basophilic cytoplasm imparting a plasmacytoid appearance (Figure 15-14).
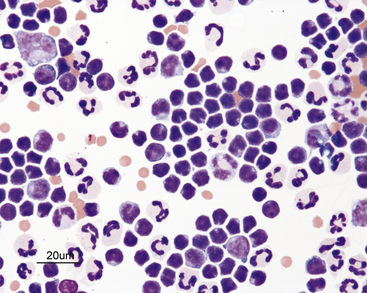
Figure 15-11 Chylous effusion from a cat.
Small lymphocytes are the predominant cells. The small lymphocytes are typically smaller than neutrophils. They have round or indented nuclei and a small amount of cytoplasm.
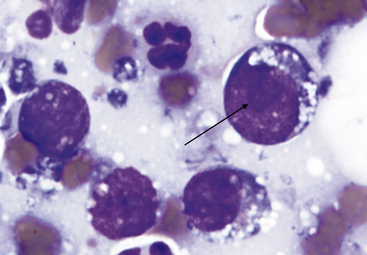
Figure 15-12 Lymphoblast with visible nucleolus (arrow). Small vacuoles are present in the cytoplasm.
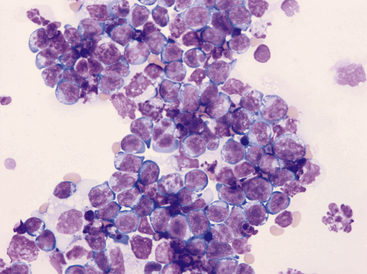
Figure 15-13 Pleural fluid from a cat with lymphoma.
Numerous lymphoblasts with visible nucleoli are present.
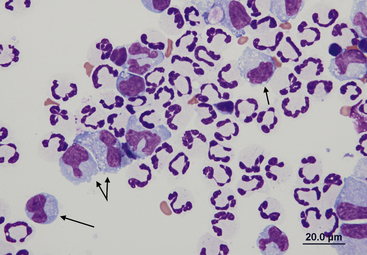
Eosinophils
Eosinophils (Figure 15-15) are readily recognized by their rod-shaped (in cats) or variably sized round (in dogs) eosinophilic granules. When present in moderate to high numbers in effusions, eosinophils are indicative of an underlying pathologic process such as heartworm disease, allergic reactions, hypersensitivity, and neoplasia (mast cell neoplasia, occult lymphoma) (Figure 15-16).12
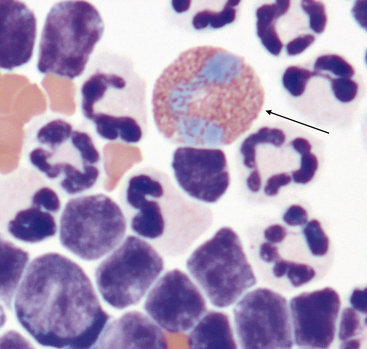
Mast Cells
Mast cells (Figure 15-17 and Figure 15-18) are readily identified by their numerous, round, red-purple cytoplasmic granules. Mast cells may be found in low numbers in effusions in dogs and cats with many different inflammatory disorders. Mast cell tumors within body cavities may be associated with effusions and frequently exfoliate large numbers of mast cells into the effusion. Visceral forms of mast cell neoplasia are rare in the dog.12,13 Visceral forms of mast cell tumors are more common in cats than in dogs and may involve the spleen, liver, gastrointestinal tract, or all of these.13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree