CHAPTER 55Early Embryonic Loss
Although not a universal definition, early embryonic loss in the mare is generally defined as pregnancy failure that occurs up to day 40 of gestation, which corresponds to the time of transition from the embryo stage to the fetal stage of conceptus development.1 The diagnosis of early embryonic loss and recognition of factors contributing to its occurrence have been dramatically improved by the widespread use of transrectal ultrasonography for early pregnancy diagnosis. Under field conditions, transrectal ultrasonography is used routinely for pregnancy diagnosis as early as day 12 to 14 postovulation, whereas under experimental conditions it may be used as early as day 10 or 11; therefore ultrasonography allows direct (and repeatable) assessment of the conceptus during approximately three quarters of the interval when early embryonic loss occurs. Before day 10 or 11 the conceptus is too small to be visualized with standard ultrasonographic equipment; therefore other techniques have been used to study embryonic loss during that interval. Specifically, assisted reproductive techniques such as embryo transfer, transvaginal ultrasound-guided follicle aspiration (TVA), and oocyte transfer and experimental techniques such as in vitro embryo culture and light/electron microscopy of oocytes/embryos have been used to study early embryonic development and embryonic loss before day 11.
INCIDENCE
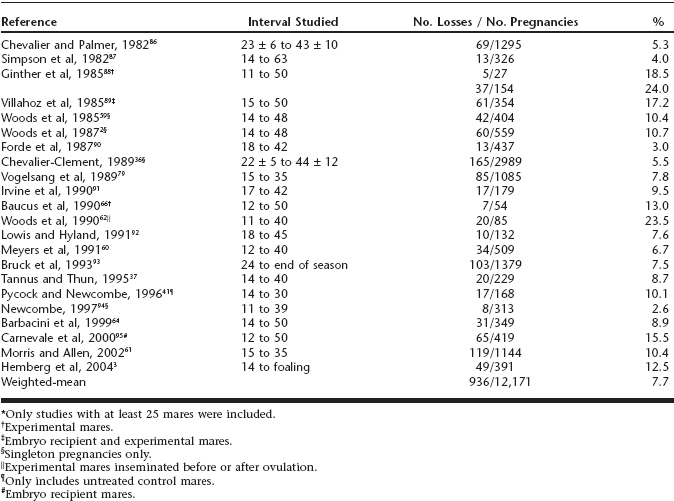
Because of the practicality and accuracy of using transrectal ultrasonography for the diagnosis of pregnancy as early as day 10, a considerable amount of data has been generated on the incidence of embryonic loss after that time. Although it can be difficult to compare data across experiments because of variables such as differences in the interval studied and whether experiments were conducted under controlled conditions or under field conditions, based on serial examination with ultrasonography the incidence of embryonic loss has ranged from approximately 2.5% to 25%, with a weighted mean across studies of 7.7% (Table 55-1). The highest embryonic loss rates (25% to 30%) have been detected in mares greater than 18 years of age.2,3
Table 55-1 A Review of Studies*on Early Embryonic Loss in Mares Detected With Transrectal Ultrasonography
The lack of a practical method of diagnosing pregnancy before day 10 makes detection of embryonic loss between fertilization and day 10 difficult. In addition, pregnancy loss before day 10 must be differentiated from fertilization failure. Data compiled from studies that used the recovery of cleaved ova on day 2 postovulation as an estimate of the fertilization rate indicate that fertilization rates were 91% in young mares and 85% in aged mares inseminated with fresh, fertile semen under experimental conditions (Table 55-2). In contrast, Carnevale and Ginther4 reported that the estimated fertilization rate on day 1.5 was significantly higher in young versus aged mares (88% versus 45%, respectively); however, that difference may have been due to delayed embryonic development in the aged mares, because the cleavage rate on day 3 was not different for young and aged mares (100% for both groups), and the embryos from aged mares were about one cleavage division delayed in their development compared with the young mares. Therefore it appears fertilization rates are very high (>90%) in young mares and may be just slightly lower (85%) in aged mares. Although fertilization rates are high, embryo recovery rates between days 6 and 9 are markedly lower for aged versus young mares,5,6 which implies a high rate of embryonic loss during the first week of gestation in the latter mares. That is further supported by reports that the estimated embryonic loss rate between fertilization and day 14 was less than 10% for young mares compared with 60% to 70% for aged mares.7,8
ETIOLOGY
Factors that may contribute to the occurrence of embryonic loss in the mare have been classified as intrinsic, extrinsic, and embryonic.9,10 Intrinsic factors include endometrial disease, progesterone deficiency, maternal age, lactation, foal-heat breeding, and time of insemination relative to ovulation. Extrinsic factors include stress; nutrition; season/climate; transrectal palpation/ultrasonography; sire and/or semen processing/handling; and gamete handling/manipulation for assisted reproductive techniques. Embryonic factors include chromoso-mal anomalies or other inherent characteristics of the embryo. Regardless of cause, indications of impending embryonic loss detectable with transrectal ultrasonography have included irregular shape of an embryonic vesicle, prolonged mobility of a vesicle, an undersized vesicle, loss of embryonic heartbeat, lack of development of the embryo proper, dislodgement of a vesicle with loss of fluid, and edema of the endometrial folds.9
Intrinsic Factors
Endometrial Disease
Endometrial disease is further classified as inflammatory or noninflammatory. Inflammatory forms of endometrial disease include acute and chronic endometritis, whereas noninflammatory forms include periglandular fibrosis and endometrial cysts. Acute endometritis is characterized by an influx of neutrophils into the stroma of the endometrium and the uterine lumen. When discussing acute endometritis, it is important to note that a physiologic (i.e., normal) acute endometritis occurs in all mares after breeding as a result of deposition of semen in the uterine lumen,11,12 and it is the spermatozoa and other components of the ejaculate/inseminate that are responsible for inducing the inflammatory response.13 Reproductively sound mares clear this mating-induced uterine contamination/inflammation between 24 and 48 hours following mating,11 whereas mares susceptible to endometritis do not,14 which leads to a persistent mating-induced endometritis. Mares that develop persistent mating-induced endometritis accumulate fluid within the uterine lumen because of impaired (i.e., delayed) physical clearance of inflammatory material14–16 exacerbated by increased fluid production. Factors that may contribute to the delayed uterine clearance in susceptible mares are (1) decreased myometrial activity,16 (2) decreased lymphatic drainage from the uterus,17 and/or (3) abnormal reproductive conformation (e.g., pendulous uterus located ventral to the pelvic brim)18 or temporary/permanent dilation of uterine horn (e.g., post partum). Similarly, mares with an acute infectious endometritis accumulate inflammatory fluid in the uterine lumen as a consequence of the body’s uterine defense mechanisms that are activated in an effort to eliminate the infectious agent.
The pathologic intrauterine fluid that accumulates during persistent mating-induced endometritis and infectious endometritis can adversely affect fertility by (1) impairing spermatozoal motility and/or viability if breeding/insemination is performed while the uterus is inflamed19,20 or (2) inducing embryonic loss if the endometritis persists beyond day 5 postovulation, when the embryo enters the uterine lumen from the oviduct21 and the corpus luteum becomes sensitive to prostaglandin F2α(PGF2α).22,23 It has been demonstrated that acute inflammation associated with intrauterine fluid collections during diestrus increases the incidence of embryonic loss.23,24 Similarly, mares with a history of endometritis had significantly higher early embryonic loss rates compared with mares with no history of endometritis.2 Therefore routine therapy for persistent mating-induced endometritis and infectious endometritis is directed at removing the fluid that has accumulated in the uterine lumen and in the case of infectious endometritis eliminating the inciting microbial agent(s). Treatment generally includes the use of ecbolic agents such as oxytocin25 and PGF2α,26 which may be used alone or in combination with large-volume uterine lavage27; in addition, appropriate antimicrobial agents are used to treat infectious endometritis.
In contrast with acute endometritis, chronic endometritis is characterized by an influx of lymphocytes into focalized areas of the endometrial stroma. Chronic inflammation appears to develop in response to any disturbance within the uterus, both normal (e.g., pregnancy) and abnormal (e.g., infectious endometritis), and is commonly identified in uterine biopsy specimens. Chronic inflammation does not appear to impair fertility, because mares with chronic inflammation can support and maintain early embryonic development.28 Therefore no treatment is indicated specifically for chronic inflammation.
Clinically, noninfectious abnormalities of the endometrium such as periglandular fibrosis have been considered an important factor in the occurrence of both early embryonic loss and fetal death,29 and because this type of pathologic change in the endometrium is generally more severe in aged mares,30,31 it seemed to be a foregone conclusion that periglandular fibrosis caused higher embryonic loss rates in aged mares. However, when Ball et al28 tested that hypothesis by transferring morphologically normal, day 7 or 8 blastocysts into the uterus of young (minimal pathologic conditions) and aged (extensive pathologic conditions) recipient mares, embryo survival rates (55% and 45% at day 12) and embryonic loss rates between days 12 and 28 (9% and 11%) were not different for the young and aged recipient mares. Their results indicated that the uterine abnormalities (periglandular fibrosis and chronic inflammation) present in the aged mare’s uteri did not result in a higher incidence of early embryonic loss in those mares; therefore it appears that other factors are primarily responsible for the higher early embryonic loss rates observed in aged mares (see discussion below).
Another common form of noninflammatory endometrial abnormality is cystic dilation, which is characterized as being either glandular or lymphatic (for review see Stanton et al32). Essentially all cysts that are grossly or ultrasonographically detectable are lymphatic in origin33 and range in size from a few millimeters to several centimeters in diameter. The incidence of endometrial cysts increases with mare age,32 which is a potentially confounding factor when assessing the effect of cysts on fertility. There are two plausible mechanisms by which cysts could adversely affect fertility. Firstly, large cysts (>3 cm) might impair intrauterine mobility of the conceptus, which could lead to failure of maternal recognition of pregnancy caused by an inability of the conceptus to adequately block endometrial PGF secretion with subsequent regression of the corpus luteum.34 Secondly, if a conceptus were to become fixed in direct contact with a cyst(s), the conceptus might be deprived of adequate nutrient exchange in a manner similar to adjacent twin conceptuses through the deprivation hypothesis proposed by Ginther.35 Several reports have described an adverse effect of endometrial cysts on fertility (i.e., decreased pregnancy rates and/or increased early embryonic loss rates)23,36,37; however, when mare age was accounted for when analyzing the data, there was no evidence of an overall effect of endometrial cysts on embryonic loss rates in mares.38
Despite the lack of conclusive evidence that endometrial cysts have a detrimental effect on fertility, treatment may be pursued in mares that have large and/or numerous cysts in conjunction with a poor reproductive history.32 Specific treatments that have been used to remove/eliminate cysts include manual rupture/ablation, drainage/aspiration, mechanical removal by ensnaring the cysts with obstetric wire, endoscopic electrocoagulation, and laser therapy (reviewed in Stanton et al32). Anecdotally, cyst removal may help some mares, because in a group of 39 aged mares (for which complete follow-up data was available) that had been barren for 1 year, 62% of the mares became pregnant after cyst removal using laser photoablation.39
Progesterone Deficiency
Despite a paucity of scientific evidence supporting its efficacy, prophylactic administration of exogenous progesterone to pregnant mares in an effort to enhance maintenance of pregnancy continues to be a widespread practice. Although low progesterone levels caused by primary corpora luteal insufficiency has been proposed as a cause of early embryonic loss in mares that could warrant administration of exogenous progesterone, its occurrence has not been clearly documented.40 Without a specific indication (or contraindication) for its use, progesterone supplementation is often empirically performed in mares that have a history of repeated pregnancy failure when no specific factor causing pregnancy loss is identified. Despite the fact that there is no evidence that routine use of exogenous progesterone will decrease the incidence of early embryonic loss, preliminary studies using the gonadotropin-releasing hormone (GnRH) agonist buserelin in mares (40 µg administered on day 10 or 11 of pregnancy) have shown a reduction in embryonic loss rate before day 30,41,42 which may be due to a beneficial effect of increased levels of endogenous progesterone.
Although primary luteal insufficiency has not been documented, it has been shown that some pregnant mares will undergo luteolysis on days 14 to 16 in spite of the presence of an embryo in the uterus.43–45 This condition is characterized by distinct edema of the endometrial folds especially in the uterine body, the vesicle is more often in the body than the horns, and the mare may begin to show signs of estrous behavior. This situation can be confirmed retrospectively by a plasma progesterone concentration of less than 1 ng/ml. The condition is most commonly seen in pregnancies conceived in the immediate postpartum period46 and raises the question of whether an abnormal embryo has failed to block luteolysis or whether spontaneous luteal regression is resulting in the loss of an otherwise viable pregnancy. The former possibility can be supported following the identification of small-for-age vesicles on days 12 to 15, which fail to block luteolysis at days 14 to 15; the mare returns to estrus and loses the vesicle. Such pregnancies may initially be saved by the timely administration of progesterone, but many of these fail to develop an embryo-proper. This is a clear example of natural elimination of a potentially nonviable pregnancy. In contrast, the clinician may sometimes find a normal size-for-age pregnancy in a mare that is returning to estrus. Prompt treatment with exogenous progesterone may prevent this impending pregnancy loss, but treatment must be continued until the mare forms an accessory corpus luteum. When the development of the vesicle and embryo appears normal, the pregnancy has a good chance of going to term.
If the decision is made to provide exogenous progesterone to a mare for maintenance of pregnancy, it is important to use one that is efficacious. Currently there are no commercially available formulations of progesterone labeled for use in pregnant mares; therefore the use of exogenous progesterone for maintenance of pregnancy is an “extra-label” use. Two preparations of progesterone commonly administered to pregnant mares are daily oral administration of the synthetic progestin altrenogest or daily intramuscular administration of progesterone in oil. Although both treatments have been shown to be efficacious by their ability to maintain pregnancy in mares without an endogenous source of progesterone,47,48 the need for daily administration is a major drawback of these formulations. In an attempt to eliminate the need for daily administration of exogenous progesterone preparations, there has been interest in the use of long-acting formulations of progesterone that can be administered at weekly (or longer) intervals. There are anecdotal reports of the clinical use of long-acting synthetic progestins labeled for use in women for maintenance of pregnancy in mares; however, these formulations were unable to maintain pregnancy in mares after PGF2α-induced luteolysis.49,50 Experimentally, progesterone-impregnated microspheres have been shown to provide sustained release of progesterone that will maintain pregnancy following PGF2α-induced luteolysis when administered every 10 days51; however, a commercially available preparation of this long-acting progesterone formulation is not available. Recently a compounded proprietary long-acting progesterone formulation was shown to be efficacious for pregnancy maintenance in mares in which endogenous progesterone secretion had been eliminated with treatment with exogenous PGF2αon day 18 of gestation.52 Administration of 1.5 g progesterone every 7 days provided sufficient progesterone levels to maintain pregnancy through day 45 of gestation, when the study was terminated.
In pregnant mares the fetal-placental unit begins secreting progesterone and related progestagens between days 80 and 100 of gestation,53 the levels of which become sufficient to maintain pregnancy after day 10054,55; therefore progesterone supplementation is generally discontinued between days 100 and 120, because there is no physiologic basis for continued administration of exogenous progesterone after that time (for a pregnancy that is developing normally).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree