Chapter 13 Drugs for the Treatment of Helminth Infections
Anthelmintics
Practicing veterinarians commonly use drugs to treat and prevent helminth infections in small animals. The life cycle and biology of the most important parasites are well understood by graduate veterinarians and are not discussed here; however, current textbooks of parasitology may be consulted for review.1–4 Gastrointestinal parasites are among the most common infectious agents that veterinarians in small animal practice face. A landmark parasite prevalence study evaluated more than 6000 canine fecal specimens from all 50 states and the District of Columbia.5 The results indicate that parasites are common in American dogs. Nationwide, 36% of the samples tested were positive for roundworm (Toxocara canis), hookworm (Ancylostoma caninum), or whipworm (Trichuris vulpis). Even more surprising, 52% of the samples from the southeastern United States were positive for at least one nematode. In a recent study of the results of heartworm and fecal testing in the western United States, the importance of annual testing and routine use of preventives was highlighted.6 Clinics in 11 states were surveyed, and local dogs with no history of travel were diagnosed with heartworms in every state but Idaho and Wyoming. The prevalence of intestinal parasites in companion animals in Ontario and Quebec, Canada, during the winter was recently evaluated.7 The fact that 30% of feline and 39% of canine fecal samples were positive for gastrointestinal parasites prompted the authors to recommend that all veterinarians follow the Companion Animal Parasite Council (CAPC) guidelines8 regarding use of year-round broad-spectrum deworming protocols. Another reason for following CAPC guidelines, in this instance regarding routine heartworm testing and prophylaxis, is concern about animals moving from heartworm-endemic areas to those with limited heartworm exposure. These concerns were realized when Hurricane Katrina resulted in thousands of dogs and cats being shipped from Louisiana, where heartworm prevalence is quite high, to shelters across the United States.9
Although these parasites are important to the health of dogs, several are also important zoonotic pathogens. Ascarid larvae migrate through human tissues, causing a variety of signs correlated to the location of the migration. These are primarily Toxocara species ascarids, but the raccoon ascarid, Baylisascaris procyonis, is being increasingly implicated as a cause of human disease in the United States.10 The Centers for Disease Control and Prevention (CDC) have published Guidelines for Veterinarians: Prevention of Zoonotic Transmission of Ascarids and Hookworms of Dogs and Cats, which is an excellent resource and is available online as a PDF download.11
Worldwide, helminth infections are a major animal and human health concern,12 with hookworms infecting large numbers of people worldwide, especially those of low economic status.13 More than 30% of the human population, in vast areas of South America and Asia, is infected with hookworms. More than half of the population is infected with hookworms in many southern areas of the African continent. Experts estimate that a billion people, more than a fifth of the planet’s human inhabitants, harbor hookworms.14 One study showed that nearly all dogs in a remote community of northeastern India were infested with one or more zoonotic gastrointestinal parasites.15 This study demonstrated that dogs played a major zoonotic role both in transmitting parasites that use dogs as their definitive and paratenic host and in mechanically transmitting and spreading the dissemination range of an array of human-specific parasites. A recent feline study in metropolitan Rio de Janeiro revealed an 89.6% prevalence of overall gastrointestinal helminth parasites in cats.16
Anthelmintics
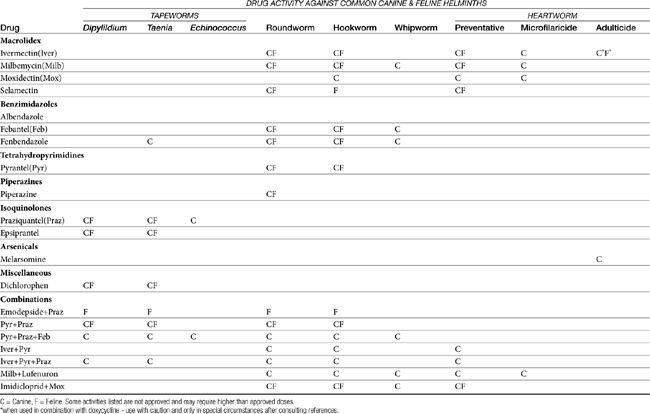
In this chapter anthelmintics that are approved by the U.S. Food and Drug Administration (FDA) and commercially available in the United States are grouped together by class according to their generic names. The literature on antiparasitic drugs is enormous. In the interest of both economy and readability, only a few references are listed for each drug. These will guide the veterinarian who needs more specific information about the subject. Table 13-1 provides a general overview of anthelmintic drug spectrum against the common canine and feline helminths.
Since the last edition of this text was published, there have been considerable changes, most notably the more widespread use of ivermectin and the emergence of other macrocyclic lactones. New information on avermectin toxicity is covered in the ivermectin section. Drug manufacturers have discontinued production of many tried-and-true anthelmintics such as dichlorvos (Task Capsules) and diethylcarbamazine citrate (Filaribits). In addition, some drugs, such as N-butyl chloride, have simply been superseded. For simplicity’s sake, discontinued products and drugs that are not widely available do not appear in the current edition; however, an earlier edition of this text can certainly be consulted for information about them. The latest information about diethylcarbamazine citrate can be obtained from the American Heartworm Society 2005 guidelines for the diagnosis, prevention, and management of heartworm infection in dogs.17 This organization produced similar guidelines for cats in 200718 and updated the guidelines for dogs in 2010.18a
New anthelmintic products are continuously researched and frequently launched. The Compendium of Veterinary Products provides a comprehensive list of commercially available products approved by the FDA.19 An exhaustive review of the pharmacology, mechanism of action, pharmacokinetics, and efficacy of anthelmintics is beyond the scope of this chapter. There are excellent texts available for those interested in more exhaustive information on anthelmintics.20–22 Pharmacologic activity against nonhelminths, such as flukes, fleas, and ticks, by some anthelmintics and anthelmintic drug combinations may be mentioned, but such activity is not the focus of this chapter.
Macrolides
Macrolides are excreted in the feces as active drug. Drugs in this class, especially the avermectins, are toxic to dung-feeding insects, but not birds, plants, and earthworms. Elimination of coprophagous insects appears to delay processing of nutrients, but the overall environmental impact of this finding is unclear.23
Although originally believed to act by disturbing gamma-aminobutyric acid (GABA)–mediated neurotransmission, it now appears that they act with high affinity to a nematode-specific glutamate-gated chloride channel.24,25 Macrolides trigger chloride ion influx, which hyperpolarizes the parasite neuron and prevents initiation or propagation of normal action potentials. The selectivity of macrolides is due to different glutamate function in invertebrates compared with that of vertebrates. Glutamate acts as an inhibitory neurotransmitter in invertebrates and an excitatory neurotransmitter in mammals.2 The net effect is paralysis and death of the target parasite.
Despite their beneficial activities, macrocyclic lactones have several flaws. They are ineffective against cestodes and trematodes, and they are sometimes expensive. That said, the U.S. patent on ivermectin has now expired, allowing generic competitors to enter the market and reduce the cost of ivermectin treatment, but to date the cost savings have not been realized on products for dogs and cats to the same degree as those for horses and food animals. Although macrolides are generally regarded as the most effective and least toxic parasiticides yet developed, toxicity may occur with overdosage, especially in Collies, many of which are unusually sensitive to macrolide endectocides. An excellent summarized overview of the pharmacology, indications, dosing, precautions, and side effects of commercially available macrocyclic lactones is available online as a PDF download from the United States Pharmacopeial Convention, Inc.23
Ivermectin
Ivermectin was the first commercially available macrolide, released for use in animals in 1981.24 The avermectins were isolated from the fermentation broth of Streptomyces avermitilis. The discovery of its anthelmintic activity was made after administration of the actinomycetic broth to mice infected with the nematode Nematospiroides dubius. Ivermectin is effective against many nematodes and arthropods. In particular, ivermectin is very effective against immature Dirofilaria immitis.
Ivermectin is well absorbed (95%) after oral administration and well distributed to most tissues except the central nervous system. It is largely eliminated unchanged in the feces and is metabolized to a small degree in the liver by oxidation. In dogs the terminal half-life is approximately 48 hours. No teratism was observed in fetuses when pregnant bitches received repeated oral doses of ivermectin of 0.5 mg/kg. A single oral dose of 2 mg/kg and repeated oral doses of 0.5 mg/kg per day for 14 weeks were well tolerated by dogs.26
Avermectin Toxicity
It is important not to administer avermectins concurrently with drugs that could increase avermectin blood–brain barrier penetration, such as ketoconazole, itraconazole, cyclosporine, and calcium-channel blockers.27 Selamectin toxicity information is addressed in detail later, in the selamectin section. According to a popular veterinary pharmaceutical clinical text, signs of ivermectin toxicity in dogs, in order of frequency, are ataxia, blindness, mydriasis, tremors, and vomiting.28 Other signs include dehydration, depression, diarrhea, hyperthermia, bradycardia, sinus arrhythmia, coma, seizures, and death.26,29,30 A recent retrospective study of ivermectin toxicosis cases evaluated at a poison control center revealed clinical signs in the following order of frequency: ataxia, lethargy, tremors, mydriasis, and blindness.30a
The apparent LD50 for Beagles is 80 mg/kg.26 The primary clinicopathologic sign in dogs is decreased serum iron values.22 A common reference used by clinical veterinarians states that death could occur with doses above 40 mg/kg, tremors at 5 mg/kg, and mydriasis at 2.5 mg/kg and that signs of toxicity rarely occur at doses below 1 mg/kg.28 But a recent retrospective poison control center study revealed that clinical signs may develop between 0.2 to 2.5 mg/kg.30a A wide variety of signs were noted, including more severe signs like coma and seizure, at doses below 1 mg/kg. In fact, death was noted in dogs that received 1 to 2.5 mg/kg doses of ivermectin.
A common presenting history is that of a dog that was in close proximity to horses during deworming and later started showing signs of disease. Dogs that develop clinical signs within 4 to 6 hours of ivermectin ingestion typically develop severe clinical signs, whereas dogs with signs developing 10 to 12 hours after exposure tend to have much milder clinical signs.29 It is not uncommon for dogs with ivermectin toxicity to have seizures. When seizures are severe and uncontrolled for a considerable period of time, hemolytic anemia and muscle damage may occur. Some severe cases present with seizures and miosis, which warrants a poor prognosis. It is possible that severe seizures and miosis on presentation may be associated with severe brain damage.31
Clinical signs of ivermectin toxicity in cats, in order of frequency, are ataxia, mydriasis, tremors, hyperesthesia, and hypothermia.28 Dogs are about 10 times as likely as cats to have ivermectin toxicity.28
Some Collies are unusually sensitive to the toxic effects of ivermectin, although it is safe for all breeds at the approved dose of 0.006 mg/kg (6 mcg/kg). Early studies indicated that some genetic lines of Collies developed severe adverse reactions when ivermectin was given at a dose of 100 to 200 mcg/kg (16 to 32 times the label dose), producing mydriasis, ataxia, tremors, drooling, paresis, recumbency, excitability, stupor, and coma. At that time Australian Shepherds, Border Collies, Shetland Sheepdogs, and Old English Sheepdogs were also reported to be sensitive to ivermectin. The lethal dose for some Collies was reported to be 1/200th that of Beagles.32
After Collies were also found to be more sensitive to loperamide,33 the canine multidrug resistance (MDR1) gene was identified and found to be mutated in ivermectin-sensitive Collies.34 MDR1 codes for a glycoprotein that is an integral part of the blood–brain barrier. There are many excellent sources of information about the MDR1 gene mutation and mechanisms of ivermectin toxicity associated with increased GABA activity.29,30,35 The mutant MDR1 allele was found in 35% of the 40 Collies that were tested in one study, about the same percentage of Collies that are sensitive to ivermectin.36 A survey of DNA from 4000 purebred dogs revealed that the MDR1 mutation was present in seven breeds of Collie lineage and two sighthound breeds, although the mutation was not identified in all breeds known to have ivermectin sensitivity.37
It was found that the potential for ivermectin sensitivity could be estimated by genotypic or polymerase chain reaction (PCR)–based testing for the MDR1 mutation.38 In this study the mutant MDR1 allele was found in Australian Shepherds, Miniature Australian Shepherds, English Shepherds, German Shepherd Dogs (white), Longhaired Whippets, McNab Shepherds, Old English Sheepdogs, Shetland Sheepdogs, Silken Windhounds, and Longhaired Whippet.23,37 Sensitivity to ivermectin was also noted in Australian Cattle Dogs, Bearded Collies, and Border Collies, but the mutant MDR1 allele was not found in these breeds.37 More recently, the relationship with P-glycoprotein, the product of the ABCB1 (formerly MDR1) gene, was studied in depth by looking for the ABCB1-1Δ allele in a DNA study of 5368 dogs.39 The ABCB1-1Δ allele was found in Collies, Longhaired Whippets, Standard and Miniature Australian Shepherds, Shetland Sheepdogs, Old English Sheepdogs, Border Collies, Silken Windhounds, and German Shepherd Dogs.
The fact is, until a particular dog is tested, its susceptibility to ivermectin toxicity is unknown. Washington State University, College of Veterinary Medicine, Veterinary Clinical Pharmacology Lab, provides genetic testing to determine the presence of the MDR1 mutant gene. Table 13-2 was adapted from information available at the aforementioned institution’s website.40 The general practitioner can also use this table when presented with a dog that has had a known exposure to ivermectin to determine prognosis and the appropriate level of treatment. As noted previously, a common finding when taking history on ivermectin and moxidectin exposure cases is that the dog was present while the owner was deworming a horse. It is not unusual for horses to spit out a small amount of dewormer, which is ingested by the dog. If the amount ingested can be quantified, then this table can help the veterinarian estimate prognosis and determine how aggressive to get with treatment. Obviously, the risk of a severe toxicity is much greater with a Collie than with another breed.
Table 13-2 Breeds Affected by MDR-1 Mutation
| Breed | Approximate Frequency |
|---|---|
| Collie | 70% |
| Longhaired Whippet | 65% |
| Australian Shepherd | 50% |
| Australian Shepherd, Mini | 50% |
| Silken Windhound | 30% |
| McNab Shepherd | 30% |
| Shetland Sheepdog | 15% |
| English Shepherd | 15% |
| German Shepherd Dog | 10% |
| Herding Breed Cross | 10% |
| Mixed Breed | 5% |
| Old English Sheepdog | 5% |
| Border Collie | < 5% |
(Data from Washington State University, College of Veterinary Medicine, Veterinary Clinical Pharmacology Lab. (2010). Affected breeds Retrieved Jan 26, 2010, from http://www.vetmed.wsu.edu/depts-VCPL/breeds.aspx.)
Another presentation to consider is dogs with the habit of eating horse feces. Dogs that are not ivermectin sensitive are probably not at risk, but an ivermectin-sensitive dog that eats the feces of a horse that has been treated with ivermectin within the last few days may have a severe, even fatal reaction. Ivermectin reaches maximum fecal concentration 2 to 3 days after the horse is treated.41 By 4 days posttreatment, 90% of the drug has been excreted in the feces. Owners of ivermectin-sensitive coprophagic dogs should treat feces from ivermectin-treated horses as toxic waste and dispose of it in a manner that will prevent the dog from eating it.
Regarding treatment of ivermectin toxicity, although there is some evidence that intravenous administration of physostigmine may be of some benefit for dogs42 and neostigmine may help treat cats43 suffering from severe ivermectin intoxication, adverse events associated with these treatments typically outweigh benefit, thus the mainstay of care given by most veterinarians is supportive and symptomatic.26 Inducing emesis, giving activated charcoal, providing fluid therapy, supplying parenteral alimentation, and maintaining respiratory support and normal body temperature are essential. This supportive care may be needed for an extended period of time because the half-life of ivermectin is 2 days and the half-life of moxidectin is 19 days.30
There is no antidote for ivermectin and moxidectin toxicity, but veterinarians should consider lipid rescue, a promising therapy adapted from human medicine. Dr. Guy Weinberg initially described the use of an intravenous lipid emulsion (Intralipid) to treat local anesthetic toxicity (bupivacaine) in humans. He coined the term “lipid rescue.” One of the studies to support human use of lipid rescue was an experiment on dogs that were overdosed with bupivacaine and rescued from certain toxicity with intravenous lipid emulsion.44 Dr. Weinberg established a noncommercial website (www.lipidrescue.com) to disseminate information and foster discussion of cases. Since then, lipid rescue has been used to treat nonbupivacaine toxicities in other species. Although support is certainly anecdotal, in 2008 a veterinary online contributor to the lipid rescue website described an ivermectin-overdosed dog that had clinical signs of toxicity and recovered nicely after activated charcoal, supportive care, and an intravenous lipid emulsion were administered. More recently, a case report of a puppy with moxidectin toxicosis was published describing the use of an intravenous lipid emulsion given as a bolus of 2 mL/kg, followed by 4 mL/kg/hr for 4 hours beginning 10 hours after exposure and repeated at 0.5 mL/kg/min for 30 minutes beginning 25.5 hours after exposure.45 The 16-week-old dog presented with acute onset seizures, paralysis, and coma soon after exposure to moxidectin. Diazepam, glycopyrrolate, and intravenous fluids were given along with respiratory ventilation and other supportive care. The puppy improved dramatically within 30 minutes of the second dose of Intralipid. Although ideal dosages have not been established, the typical recommendation is for bolus administration of 1.5 mL/kg of intravenous lipid emulsion, followed by 0.25 mL/kg/min for 30 to 60 minutes.46 Other brands of intravenous lipid emulsion, such as Liposyn, can also be considered. It is best to have the product available ahead of time rather than try to acquire it in the midst of an emergency. Dr. Weinberg’s lipid rescue website (mentioned previously) describes preparation of a kit to have on hand.
Dogs
Ivermectin (Heartgard) tablets are administered orally at a dose level of 0.006 mg/kg (6 mcg/kg) at monthly intervals to prevent the establishment of the D. immitis. The initial dose should be given within 1 month of the first exposure to mosquitoes and throughout the period of the year when mosquitoes are active. The last treatment must be given to dogs within 1 month after the last exposure to mosquitoes. Ivermectin alone has minimal activity against the adult heartworm in the short term. It is active on the third- and fourth-stage larvae and the circulating microfilariae. A single oral dose of ivermectin administered within 2 months of infection prevents the establishment of adult worms in the heart. A single oral dose of 0.05 mg/kg is adequate to clear the circulating microfilariae when given to dogs 4 weeks after the administration of an adulticide, although ivermectin is not approved as a microfilaricide.47 Review of the original reference is suggested for more complete information. When ivermectin (0.006 mg/kg) is given to heartworm-positive dogs over several months, the circulating microfilariae are eliminated, resulting in an occult infection. Thus dogs receiving monthly ivermectin should be tested annually with an occult heartworm test.17,48,49
Knight provides an excellent review of heartworm testing and suggested chemoprophylaxis timing for various regions in the United States.50 The American Heartworm Society guidelines for diagnosis, prevention, and management of heartworm infection in dogs should also be consulted.17 Although there is no FDA-approved microfilaricide, macrocyclic lactones are the safest and most effective microfilaricidal drugs available for use in heartworm-positive dogs.17 Compared with ivermectin, milbemycin is a more potent microfilaricide and causes quicker clearance of microfilariae.17
Short-term use of ivermectin alone has minimal effect on adult heartworms, but when given continuously over a prolonged period, for 1 to 2 years, or when combined with doxycycline, it may have some utility for treating dogs with adult heartworm infection. The older the adult heartworms are when first exposed to ivermectin, the longer it takes them to die; because they continue to cause damage during this time, long-term ivermectin therapy generally is not a substitute for melarsomine (Immiticide) therapy.17 In addition, a mild hypersensitivity reaction has been observed in dogs with circulating microfilariae that are treated with ivermectin. Many products that contain ivermectin have precautions suggesting removal of adult heartworms and microfilariae before initiating ivermectin heartworm prophylaxis.
Regarding the combination of ivermectin and doxycycline as a heartworm adulticide; it has been found that Wolbachia spp. bacteria are filarial species endosymbionts—that is, their presence is necessary for filial worm survival—and that eliminating this bacteria from heartworm-positive dogs and cats will decrease host antigenic response.51,52 In fact, one study of heartworm-positive dogs comparing groups that were treated with three drugs (i.e., melarsomine, doxycycline, and ivermectin), two drugs (i.e., doxycycline and ivermectin), doxycycline alone, ivermectin alone, and melarsomine alone, led the authors to conclude that the combination of doxycycline and ivermectin was synergistic and could eliminate adult heartworms with less potential for severe thromboembolism than melarsomine alone.52 This is discussed in greater depth in the melarsomine section.
Ivermectin given as a single subcutaneous injection or orally administered at 0.2 mg/kg demonstrated high efficacy against the immature and adult T. canis, A. caninum, Ancylostoma braziliense, Uncinaria stenocephala, Strongyloides stercoralis, Capillaria spp., and Filaroides hirthi.53 At that dose its activity against Toxascaris leonina and T. vulpis is erratic.23 When treating respiratory nematode parasites a higher dose, 0.4 mg/kg by subcutaneous injection or orally every 2 weeks for 2 to 3 doses has been recommended recently for Oslerus (Filaroides) osleri, F. hirthi, Aelurostrongylus abstrusus, and Capillaria aerophila infections.54
Cats
Ivermectin is FDA approved as a monthly heartworm preventive in cats (Heartgard Chewable for Cats). The approved monthly oral dose of 0.024 mg/kg is effective in preventing the development of D. immitis and hookworms (A. braziliense and Ancylostoma tubaeforme).55–57 Feline roundworm (Toxocara cati) infections have been controlled with 0.2 to 0.3 mg/kg of ivermectin and lungworm (A. abstrusus) infections with 0.4 mg/kg of ivermectin.58–60 Capillaria species are rarely implicated in feline cystitis, and infestations are thought to be self-limiting usually, but in one case a single dose of ivermectin 0.2 mg/kg, administered subcutaneously, was successfully used to treat the condition in a cat.61
Milbemycin Oxime
Dogs
Milbemycin oxime tablets (Interceptor) are formulated to deliver a minimum dose of 0.5 mg/kg of body weight. When given every 30 days it is effective in preventing heartworms (D. immitis).62–64 It also kills A. caninum, T. canis, and T. vulpis.65–6823 One study indicates that milbemycin may help control raccoon roundworm (B. procyonis) infections in dogs and thus decrease the zoonotic potential of a parasite that can have devastating effects in humans, including death.69
Milbemycin oxime has been extensively tested with regard to safety. It is nontoxic to Collies at up to 20 times the recommended dose and is safe when given to pregnant and nursing animals.70,71 Milbemycin oxime, like ivermectin, is known to kill heartworm microfilariae and inhibit the release of new microfilariae. Thus all dogs receiving routine monthly heartworm prophylaxis with milbemycin should be tested with adult antigen tests.72–74,49,17
Moxidectin
KEY POINT 13-11
Moxidectin, the third macrolide used commercially, is also similar to ivermectin and milbemycin.
Dog
Moxidectin injection (ProHeart 6) was launched in the United States in 2001 with an indication to prevent heartworms and treat hookworms in dogs. The label for the sustained-release injectable product instructed that it was to be given no more often than once every 6 months. The FDA had concerns about safety as a result of adverse event reports that it received, and the manufacturer voluntarily recalled the product from the U.S market in 2004 to address those safety concerns.77 During that time the product remained on the market in Australia, Japan, and parts of Europe. In 2008 the product was reintroduced to the U.S. market with a new label under a postmarketing surveillance initiative based on human drug programs and known as a Risk Minimization Action Plan (RiskMAP), which includes veterinarian training and use of a pet-owner consent form. This is the first veterinary drug to be marketed under RiskMAP, a strengthened risk minimization and restricted distribution program.78 The new label advises not to administer the drug to sick, debilitated, or underweight dogs or those with a history of weight loss and states that the product should be used with caution in dogs with preexisting allergic disease, including food allergy, atopy, and flea allergy dermatitis. The label also warns not to administer moxidectin injection within 1 month of vaccinations.79
Injectable moxidectin is indicated for use in dogs 6 months of age and older for the prevention of heartworm disease. It should be given at 0.17 mg/kg by subcutaneous injection within 1 month of the dog’s first exposure to mosquitoes or within 1 month of the dog’s last dose of monthly heartworm preventive. The sustained-release injection provides a 6-month window of protection from heartworms.79 However, it does not clear microfilariae or remove adult heartworms.23 A challenge study comparing efficacy and adverse reactions among four groups of dogs given placebo or moxidectin at 0.06, 0.17, or 0.5mg/kg and inoculated with 50 D. immitis third-stage larvae 180 days later revealed 100% efficacy at the label dose (0.17 mg/kg).80 However, one of eight dogs given the lower dose (0.06 mg/kg) was infected. The authors speculated that the failure of protection was a result of individual pharmacokinetic variation because the moxidectin serum concentration in the unprotected dog was at the limit of quantitation (lowest detectable quantity) 8 days after treatment and undetectable thereafter compared with others in that group that had detectable concentrations until at least day 14 and for as long as 55 days for most of the other dogs.80 Both the frequency and size of injection-site granulomas correlated positively with the moxidectin dose.80
Selamectin
Prepared by semisynthetic modification of doramectin,81 selamectin (Revolution) is the latest macrolide to enter the U.S. marketplace. This product is unique among other macrolides used in small animals in that it is formulated for convenient topical administration. It is simply “spotted” onto the skin of the pet. It was the first macrocyclic lactone approved for use in dogs and cats to provide activity against both internal and external parasites. However, the discussion here focuses on the activity of selamectin against internal parasites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree