Chapter 13 Disorders of Micturition and Urinary Incontinence
Introduction
A. Micturition is the process of urinary storage (filling) and voiding (emptying). Approximately 99% of the time, the bladder and urethra are occupied in the storage of urine whereas only 1% of the time is occupied with voiding.
B. Incontinence is the involuntary passage of urine. The affected animal typically appears to be unaware of urine leakage, at least initially. Increased grooming of the perineum suggests awareness of the problem at some point in its clinical course.
D. Urinary incontinence is much more common in dogs than in cats. When encountered in cats, urge incontinence from cystitis must be excluded as the most likely consideration.
F. Most forms of incontinence occur only when bladder pressure exceeds urethral resistance pressure. Incontinence may occur when bladder pressure is abnormally high (transiently or persistently), urethral pressure is low, or a combination of these factors. Exceptions to this rule occur when the incontinence is due to anatomical defects (e.g., ectopic ureter) that bypass the normal urethral sphincter mechanism.
Physiology of Urination
A. The normal physiology of urination involves both voluntary and involuntary (i.e., autonomic) components of the nervous system.
B. Stretch receptors in the bladder carry afferent information (general visceral afferent [GVA] and general proprioceptive [GP]) about bladder distension to the spinal cord.
C. This information is relayed to higher central nervous system (CNS) centers (e.g., brainstem, cerebral cortex) via the spinothalamic tract and general proprioceptive tract in the fasciculus gracilis allowing conscious perception of bladder distension.
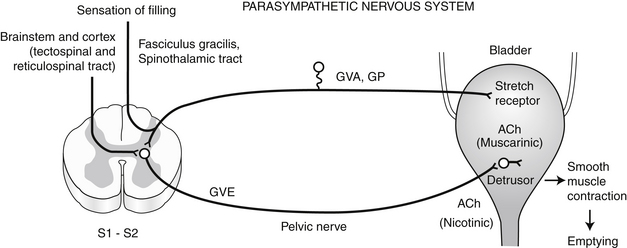
D. If the circumstances are appropriate for urination, the emptying phase is initiated. The parasympathetic portion of the autonomic nervous system plays the dominant role in the emptying phase (Figure 13-1).
1. Motor information descends from the higher CNS centers via the tectospinal and reticulospinal tracts to parasympathetic general visceral efferent lower motor neurons in the gray matter of the sacral spinal cord (S1-S2).
2. These neurons reach the detrusor muscle of the bladder via the pelvic nerve and initiate detrusor contraction and bladder emptying.
3. This control is mediated by the parasympathetic nervous system and neurotransmission is cholinergic at both the preganglionic and postganglionic fibers. The receptors at the smooth muscle of the detrusor are muscarinic.
4. The GVA/GP afferent neurons from the bladder wall also synapse on the parasympathetic general visceral efferent (GVE) lower motor neurons in the sacral spinal cord. This is the basis for local spinal cord reflex voiding (see later).
5. At the same time, efferent fibers from higher centers inhibit transmission in the sympathetic nervous system, which normally facilitates bladder filling.
6. Also, there is inhibition of general somatic efferent (GSE) neurons located in the sacral spinal cord (S1-S3) that exit in the pudendal nerve and normally maintain tone in the striated muscle of the urethra (so-called external urethral sphincter). Failure of this inhibition contributes to increased urethral sphincter tone in patients with upper motor neuron (UMN) bladders.
7. GSE neurons to the striated muscle of the abdomen are stimulated and initiate abdominal wall contractions that increase intra-abdominal pressure and are important in the voiding response. This somatic component represents part of the voluntary control of urination.
8. Injury that results in a CNS lesion cranial to the sacral spinal cord is associated with detrusor areflexia and increased urethral sphincter tone (due in part to loss of UMN inhibitory influence).
c. Within a few weeks of injury, local reflex voiding develops and is associated with incomplete bladder emptying and abnormally high residual volume.
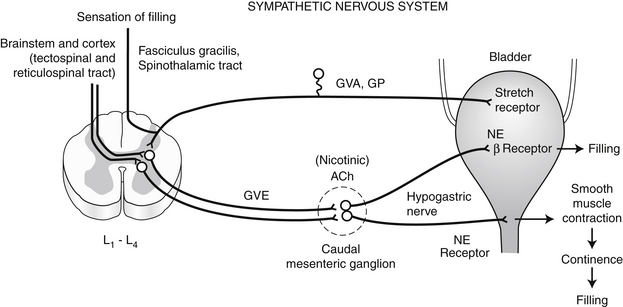
E. If the circumstances are inappropriate for urination, the filling phase continues. The sympathetic portion of the autonomic nervous system plays the dominant role in the filling phase (Figure 13-2).
1. Efferent fibers descending from higher centers in the CNS inhibit the GVE lower motor neurons of the parasympathetic nervous system that are located in the sacral spinal cord (S1-S2).
2. Sympathetic GVE neurons in the lumbar spinal cord (L2-L5) are stimulated and these preganglionic fibers synapse at the caudal mesenteric ganglion (cholinergic mediation). The postganglionic adrenergic fibers reach the bladder wall via the hypogastric nerve.
3. The postganglionic fibers that reach the body of the bladder terminate primarily on beta-adrenergic receptors. When these receptors (found primarily in the body of the bladder) are stimulated there is relaxation of the detrusor smooth muscle and bladder filling is facilitated.
4. The postganglionic fibers that reach the bladder neck and proximal urethra terminate mainly on alpha-adrenergic receptors. When these receptors (found primarily in the bladder neck and proximal urethra) are stimulated, contraction of smooth muscle in these regions occurs, which facilitates bladder filling and maintains continence. The smooth muscle in the proximal urethra is referred to as the internal urethral sphincter.
5. The distribution of beta- and alpha-adrenergic receptors in the bladder and proximal urethra (see earlier) establishes the role of the sympathetic nervous system in the regulation of urination.
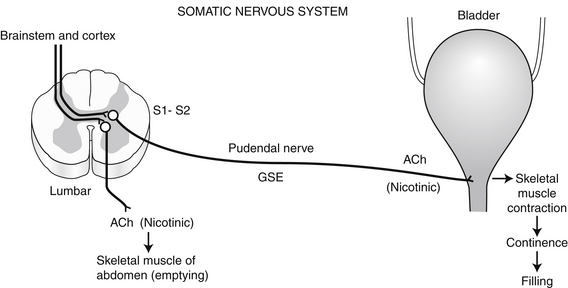
6. Also during bladder filling, efferent fibers descending from higher CNS centers stimulate GSE neurons of the sacral spinal cord (S1-S2) that travel in the pudendal nerve to innervate the striated muscle of the urethra, the so-called external urethral sphincter. Neurotransmission at this striated muscle is cholinergic via nicotinic receptors. This somatic component represents part of the voluntary control of urination (Figure 13-3).
F. In summary, afferent information from the bladder about its state of distension ultimately reaches higher CNS centers in the brainstem and cerebral cortex. The higher CNS centers integrate this information with other afferent input and institute the appropriate efferent response with respect to urination.
G. It is important to understand how the GVE neurons of the parasympathetic and sympathetic components of the autonomic nervous system and the GSE neurons of the somatic nervous system interact in the control of bladder filling and emptying because the drugs that are used to manage animals with disorders of urination act on these systems.
H. Even a bladder that has lost its local sacral reflex arc consisting of the GVA and GVE parasympathetic neurons in the sacral spinal cord and pelvic nerve eventually can develop the ability to empty partially.
3. Such a bladder is called a lower motor neuron (LMN or autonomous) bladder and results from lesions in the sacral spinal cord, cauda equina, or pelvic nerves. The caudal vertebral and spinal cord malformations that occur in Manx cats represent a congenital condition causing a LMN bladder.
4. The residual volume of urine in a LMN bladder usually exceeds that in an UMN bladder. Increasing the animal’s intra-abdominal pressure by picking it up may initiate involuntary voiding.
Differential Diagnosis for Urinary Incontinence (Box 13-1)
A. The causes of urinary incontinence classically are divided into neurogenic or non-neurogenic categories. Non-neurogenic causes are most common, and a thorough neurological examination is important in making this distinction.
Clinical Approach to Patients Presented for Evaluation of Abnormal Micturition
Signalment
A. Congenital disorders are most commonly recognized in young animals. Certain large breed dogs are at increased risk for ectopic ureters. Manx cats have urinary incontinence associated with congenital vertebral and spinal cord anomalies.
B. Hormone-responsive incontinence and urethral sphincter incompetence usually occur in middle-aged, medium to large breed female dogs; certain breeds are at increased risk for this disorder (see later).
History
A. It is important to differentiate loss of voluntary control from behavioral changes or recent onset of polyuria and polydipsia.
1. Development of polyuria as a result of an underlying disease such as chronic renal failure may result in the animal urinating in the house because it is not being allowed outdoors frequently enough.
B. It is important to question the owner about the presence of dysuria or hematuria suggestive of urinary tract infection (UTI), inflammation or partial urinary tract obstruction.
C. It is important to identify any previous episodes of trauma or surgery adjacent to or involving the urinary tract that could have affected micturition.
Physical Examination
A. Observe the animal urinating. Characterize the animal’s ability to initiate a urine stream, the diameter of the stream, any interruptions of the stream, and any apparent pain.
2. Determine the presence or absence of pain associated with palpation of the bladder (and prostate gland in males).
3. Determine bladder size after voiding. An enlarged bladder after attempts to void suggests urine retention.
4. Identify any masses in the bladder or prostate gland in males that could be associated with outflow obstruction, inflammation, or altered function.
D. Depending on the clinical circumstances, digital vaginal examination in female dogs can be performed to evaluate for presence of a mass in the region of the vestibule or external urethra.
E. Perform a complete neurologic examination to assess the cerebrum, brainstem, and spinal cord. Pay special attention to the local sacral reflex arc including:
2. Bulbocavernosus reflex (manual compression of the bulbus glandis or clitoris causes contraction of the anal sphincter via the pudendal nerve).
F. In animals with incontinence and an enlarged bladder, passage of a urethral catheter to rule out intraluminal obstruction can be helpful. Extramural masses may not be identified by this procedure.
Urinary Tract Imaging
A. Urinary tract imaging is performed if the diagnosis is not apparent after initial evaluation or if empirical treatment has not been effective.
C. Contrast radiography is helpful to rule out anatomic abnormalities or radiolucent calculi.
1. Excretory urography can be used to evaluate for the presence of ectopic ureters (see later), ureteral dilatation, or dilatation of the renal pelvis.
E. Urethrocystoscopy is helpful to evaluate the vestibule, vagina, urethra, and bladder and to identify abnormal ureteral openings, masses, and other anatomic abnormalities (e.g., urachal diverticulum).
Special Studies
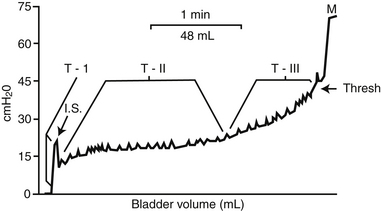
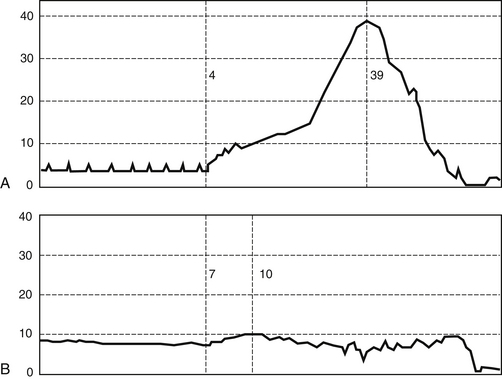
A. The cystometrogram (CMG) is a pressure-volume recording of the bladder’s response to filling with fluid or CO2 (Figure 13-4).
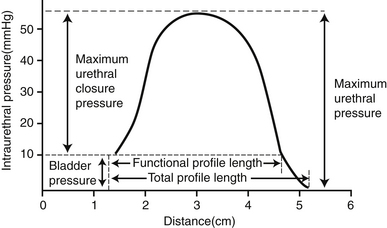
B. The urethral pressure profile (UPP) is a pressure tracing of the urethra as a catheter is slowly withdrawn from the bladder at a constant speed (Figures 13-5 and 13-6).
D. The UPP and CMG are not needed to establish a presumptive diagnosis in most dogs with urinary incontinence.
F. FPL is the most consistent parameter on the UPP. MUCP is more variable. MUCP occurs in the middle to distal urethra of female dogs and cats and in the postprostatic urethra of male dogs and cats.
Abnormal Urination (Disorders of Micturition)
Non-neurogenic Causes of Urinary Incontinence
Primary Sphincter Mechanism Incompetence (PSMI)
a. PSMI is the most common non-neurogenic cause of urinary incontinence in dogs.




(1) Incontinence in spayed female dogs previously was called hormone-responsive, or estrogen-responsive, incontinence.
(2) Occurs approximately 3 years after ovariohysterectomy in approximately 20% of female dogs neutered between their first and second heat cycles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree