Chapter 9 Disorders of Bone Marrow
Generalized Increases in Hematopoietic Cells
Bone marrow is generally considered to be hypercellular when more than 75% of the space consists of hematopoietic cells.168 This estimate is easily performed in core bone marrow biopsy specimens, but is more challenging in bone marrow aspirate smears that have variable amounts of blood contamination. In aspirate smears, the cellularity is generally determined by examining as many marrow particles (spicules) as possible and estimating the area occupied by cells versus the area occupied by fat.
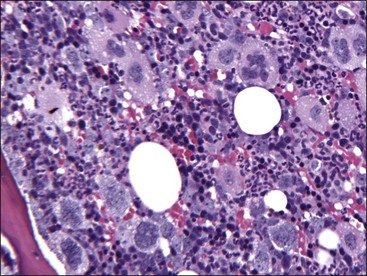
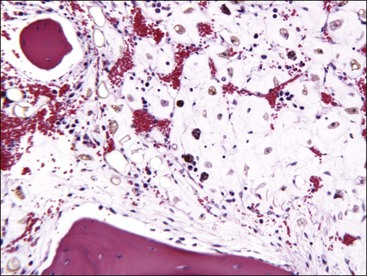
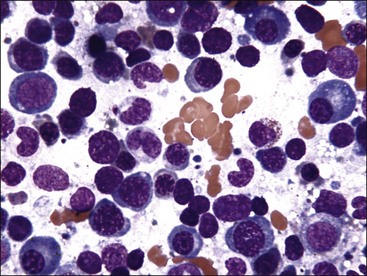
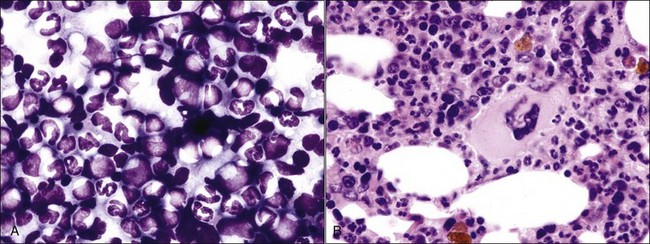
Marrow can become hypercellular when one or more cell lines exhibit increased proliferation in response to peripheral needs or demands. For example, both erythrocytic and granulocytic cell lines may be increased in immune-mediated hemolytic anemia (IMHA) in dogs, in which animals often exhibit a regenerative anemia with accompanying leukocytosis and left shift.409 Megakaryocytic hyperplasia can occur if immune-mediated thrombocytopenia (IMT) is present. In a retrospective study of dogs, panhyperplasia of bone marrow was observed most frequently in association with mast cell tumors, lymphomas, and blood loss anemia.508 Hypercellularity was exhibited in bone marrow from a dog that had blood-loss iron deficiency anemia with an associated thrombocytosis (Fig. 9-1) and from a dog that had inherited erythrocyte phosphofructokinase deficiency with an accompanying neutrophilia (Fig. 9-2). Panhyperplasia has been reported in the bone marrow of cats with IMHA, IMT, and feline leukemia virus (FeLV)-associated anemia.507 Although rare, generalized marrow hyperplasia may occur in response to cytopenias in blood resulting from hypersplenism.423,523 Generalized hypercellular marrow may be present in some animals with myelodysplastic disorders, but abnormalities in cell morphology and/or distributions are present (see Fig. 8-36, A-C). Primary erythrocytosis (polycythemia vera) may sometimes exhibit a generalized marrow hyperplasia.231,500
Generalized Decreases In Hematopoietic Cells
Hypocellular/Aplastic Bone Marrow
Bone marrow is generally considered to be hypocellular when less than 25% of the space consists of hematopoietic cells.168 This estimate is easily performed in core bone marrow biopsy specimens but is more challenging in bone marrow aspirate smears that have variable amounts of blood contamination. In aspirate smears, the cellularity is generally determined by examining as many marrow particles as possible and estimating the area occupied by hematopoietic cells versus the area occupied by fat. However, it should be recognized that fat may not have replaced hematopoietic cells in some disorders, including acute marrow injury with necrosis and edema, gelatinous transformation of bone marrow, and myelofibrosis.
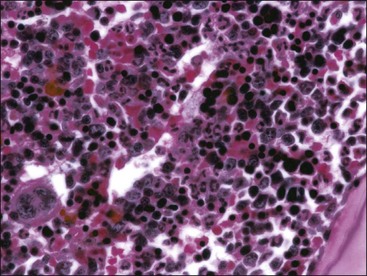
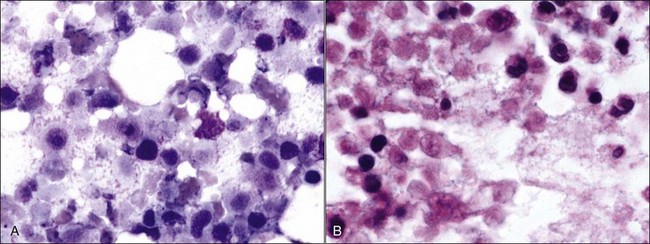
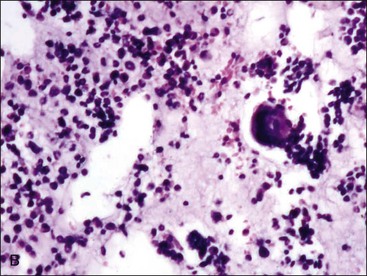
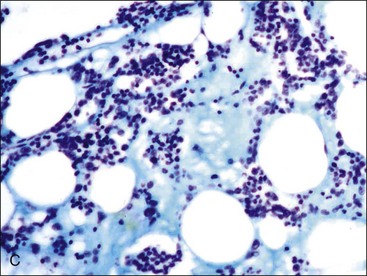
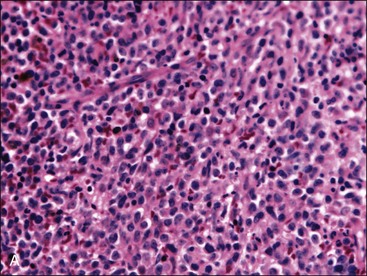
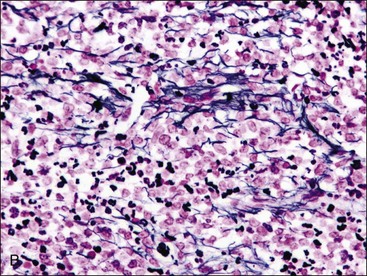
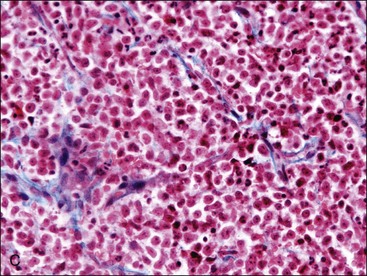
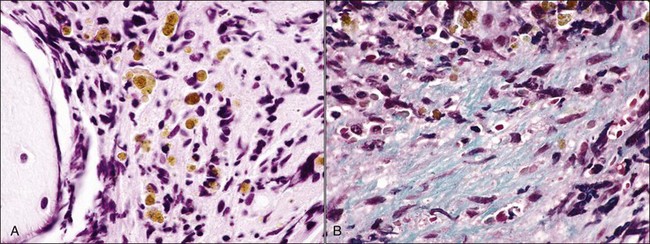
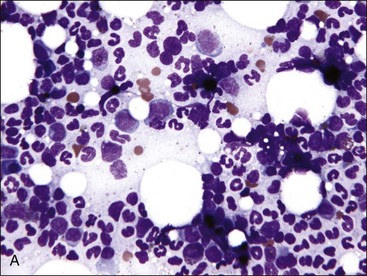
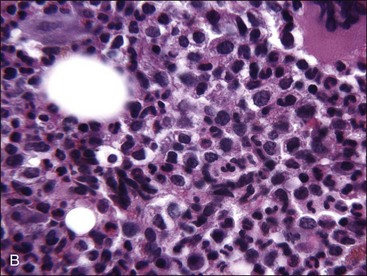
When all hematopoietic cell types—erythrocytic, granulocytic, and megakaryocytic—are markedly reduced or absent, the marrow is said to be aplastic; anemic animals with generalized marrow aplasia are said to have an aplastic anemia (Fig. 9-3, A,B). Stromal cells (adipocytes, reticular cells, endothelial cells, and macrophages), plasma cells, and some lymphocytes are still present in aplastic bone marrow samples (Fig. 9-3, C). Macrophages typically contain increased amounts of hemosiderin because storage iron is not utilized for erythrocyte production (Fig. 9-3, C,D). Mast cells may also be present in moderate numbers in aplastic bone marrow samples in dogs (Fig. 9-3, C).42,332,489 The peripheral blood is characterized by a nonregenerative anemia, neutropenia, and thrombocytopenia.
The majority of cases of aplastic anemia in humans are idiopathic; however, viruses, chemicals, and idiosyncratic reactions to certain drugs have been associated with some human cases.228 Most cases of aplastic anemia in humans appear to be mediated by a T lymphocyte immune reaction against hematopoietic stem cells. A drug or virus may trigger the expansion of cytotoxic T lymphocytes, resulting in the destruction of hematopoietic stem cells. Interferons (especially interferon-γ) and tumor necrosis factor-α (TNF-α) produced by these cells can induce apoptosis in CD34+ hematopoietic progenitor cells, which may contribute to hematopoietic suppression in humans with aplastic anemia.550 A primary immune-mediated reaction directed against hematopoietic precursor cells has also been proposed as a cause of aplastic anemia in dogs.489
Drug-induced causes of aplastic anemia or generalized marrow hypoplasia in animals include estrogen toxicity in dogs438; phenylbutazone toxicity in dogs498,527 and possibly a horse104; trimethoprim-sulfadiazine administration in dogs130,515,527; bracken fern poisoning in cattle and sheep354,432; trichloroethylene-extracted soybean meal in cattle449; albendazole administration in dogs, cats, and juvenile alpacas159,448,522; fenbendazole administration in a dog142; griseofulvin toxicity in cats and possibly a dog47,178,402; azathioprine toxicity198,393,515; various cancer chemotherapeutic agents363,400,501,523; and radiation.339,417,418 Meclofenamic acid and quinidine have also been incriminated as potential causes of aplastic anemia in dogs.527 Other drugs are believed to be myelosuppressive, causing multiple cytopenias, but bone marrow was not evaluated to demonstrate aplasia.515
Exogenous estrogen injections can result in aplastic anemia in dogs, as can high levels of endogenous estrogens produced by Sertoli cell, interstitial cell, and granulosa cell tumors.302,325,427,450 Functional cystic ovaries also have the potential of inducing myelotoxicity in dogs.55 Ferrets have induced ovulations and may remain in estrus for long periods of time when not bred. This prolonged exposure to high endogenous estrogen concentrations can result in aplastic anemia.30,236
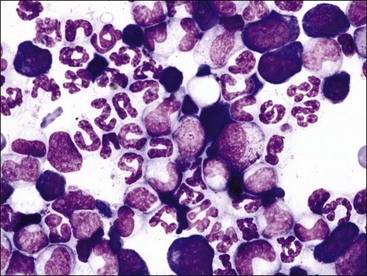
Parvovirus infections can cause erythroid hypoplasia as well as myeloid hypoplasia in canine pups,370,395 but the animals may not become anemic because of the long life spans of erythrocytes. Thrombocytopenia is mild or absent because megakaryocytes may still be present in the bone marrow (Fig. 9-4, A,B). Either affected pups die acutely or the bone marrow returns rapidly to normal before anemia can develop. In contrast to its effects in pups, parvovirus is reported to have a minimal effect on erythroid progenitors in adult dogs.54 Only myeloid hypoplasia was reported during histologic examination of bone marrow from parvovirus-infected viremic cats in early studies252,255; however, in a later study, generalized marrow aplasia has been reported in naturally infected cats that died.53
Although some degree of marrow hypoplasia and/or dysplasia often occurs in cats with FeLV infections,86 true aplastic anemia is not a well-documented sequela.399 Hypocellular bone marrow has been reported in experimental cats coinfected with FeLV and feline parvovirus.277
Aplastic anemia with resultant pancytopenia and depletion of lymphoid tissues has been reported in neonatal calves in Europe. Prominent clinical signs included mucosal petechial hemorrhages, cutaneous bleeding, melena, and high fever. A viral etiology was suspected, but viral isolation was not successful. Polymerase chain reaction (PCR) assays for bovine viral diarrhea (BVD), bluetongue, and epizootic hemorrhagic disease virus were also negative.352
Dogs with acute Ehrlichia canis infections may recover spontaneously or develop chronic disease, which generally involves some degree of marrow hypoplasia. Although rare, aplastic anemia may develop in association with severe chronic ehrlichiosis in dogs.59,331 Erythroid and megakaryocytic hypoplasia has been reported in two cats with E. canis-like infections.50
Hypocellular bone marrow has been reported in a dog with splenomegaly and marked extramedullary hematopoiesis, which returned to normal after splenectomy.244 It was speculated that the spleen might have produced cellular or humoral inhibitors of hematopoiesis in the bone marrow.
Congenital aplastic anemia, renal abnormalities, and skin lesions have been reported in newborn foals whose dams were treated for equine protozoal myeloencephalitis with sulfonamides, pyrimethamine, folic acid, and vitamin E during pregnancy.468 Aplastic anemia was present at birth in a foal born to a mare that was treated with trimethoprim-sulfamethoxazole for severe placentitis for 1 to 2 months before she gave birth (Fig. 9-5). Aplastic anemia in a 14-day-old Holstein calf may have also developed in utero, although the calf was treated for diarrhea with sulfamethazine 5 days before examination.10 An in utero toxic insult was suspected in a 9-week-old Clydesdale foal with aplastic anemia.317
Generalized bone marrow hypoplasia, with myeloid and megakaryocytic hypoplasia more prominent than erythroid hypoplasia, has been reported in eight young standardbred horses sired by the same stallion.237 Genetic defects involving the marrow microenvironment, one or more growth factors, or pluripotent stem cells were suggested as possible causes. Hypocellular bone marrow has been reported in a Holstein calf with congenital chondrodysplastic dwarfism.337
Idiopathic aplastic anemia has also been reported in dogs,111,489,521 cats,507 and horses.29,258,317 One case of erythroid and myeloid aplasia with normal megakaryocyte numbers has been reported in a horse; the etiology was unknown.493
Acute Bone Marrow Injury and Necrosis
Two major forms of cell death, necrosis and apoptosis, are recognized. Necrosis refers to a form of cell death and degeneration secondary to the inability of mitochondria to generate sufficient energy in the form of ATP. Cells swell and burst after losing their ability to regulate osmotic balance. A variable inflammatory response occurs secondary to the release of intracellular contents. In contrast, mitochondrial function is less affected and ATP concentrations are higher in cells undergoing apoptosis (physiologic cell death). The nuclear chromatin condenses, the nucleus rounds up into a single dense sphere (pyknosis) or fragments into multiple dense spheres (karyorrhexis), and the cell shrinks by as much as 30%. Soon after the process is begun, the cell is recognized and phagocytized by macrophages. Cytoplasmic contents are not shed externally; therefore, there is no release of proinflammatory mediators. A third form of cell death, called autophagy-associated cell death, occurs when cells do not receive sufficient nutrients for extended periods of time and therefore digest available internal substrates and die.196
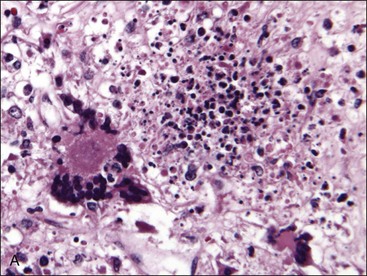
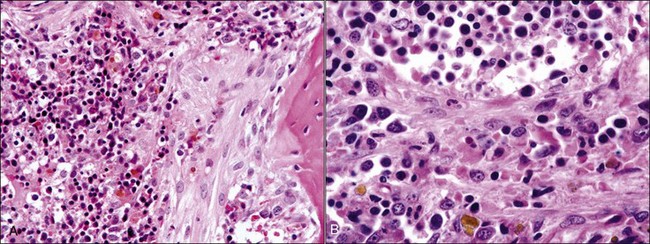
Necrosis may be caused by ischemia resulting from damage or disruption of the microcirculation or by direct damage to the proliferating hematopoietic cells. Edema (amorphous proteinaceous material between hematopoietic cells), hemorrhage, and acute inflammation may also be present as a result of increased vascular permeability following vessel injury.517 Necrosis may be recognized antemortem (Fig. 9-6, A), but it is most often recognized when histologic samples are examined postmortem (Figs. 9-6, B, and 9-7, A) because it is a transient event that often has a focal distribution.
The appearance of necrosis varies depending on the time course and cause.195 When histologic sections are examined, initial lesions exhibit altered staining of hematopoietic cells with indistinct cellular outlines (see Fig. 9-6).500 Hemorrhage and/or edema may also be present if vessels are injured.195 Later, the areas of necrosis become hypocellular as the cells lyse and are replaced by amorphous granular eosinophilic debris. This stage of necrosis must be differentiated from fibrin, edema, and collection artifact. A Fraser-Lendrum stain for fibrin can be helpful in this regard.500 Macrophages, many of which contain phagocytized cellular debris, occur in increased numbers in necrotic marrow. Myelofibrosis occurs subsequently to necrosis, similar to the healing process that occurs in other damaged tissues.188,195,513
Aspirate smears from necrotic marrow may be confused with smears resulting from poor-quality sample collection or staining techniques. Marrow particles may appear elongated and “stringy.”520 The background appears granular and is bluish to purple in color. The remaining cells are difficult to classify because of morphologic changes caused by degeneration (see Fig. 9-6, A). Nuclei appear smudged and cytoplasmic margins are usually ill defined. When visible, the cytoplasm is basophilic and sometimes vacuolated.188,391 Often only free nuclei or nuclear fragments are seen. Macrophages with phagocytized debris are commonly observed.119,520
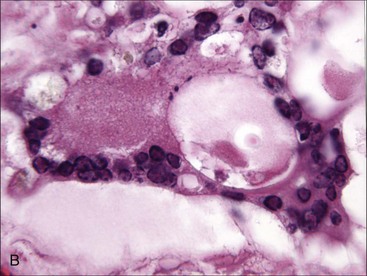
Disorders that have been reported in association with marrow necrosis in animals include septicemia and/or endotoxemia,503,520,523 feline infectious peritonitis,53,510 FeLV infection,430 acute parvovirus infection in dogs,43 BVD,112,415 Ehrlichia canis infection in dogs,520 drug administration (including chemotherapeutic agents, estradiol [dogs], phenobarbital, mitotane, carprofen, metronidazole, colchicine, fenbendazole, and cephalosporin antibiotics),36,188,199,503,517 neoplasia,101,114,227,500,503 myelodysplastic syndrome,507 nonregenerative IMHA,512 systemic lupus erythematosus in dogs,119,503 disseminated intravascular coagulation,388,519,523 and chronic renal disease in cats treated with recombinant erythropoietin (EPO) or blood transfusions.510 Multifocal bone marrow necrosis and fibrosis was recognized in a horse with an equine herpesvirus-2 infection (see Fig. 9-7). The cause of bone marrow necrosis may not always be identified.120,188,503,507,529
Gelatinous Transformation of Bone Marrow
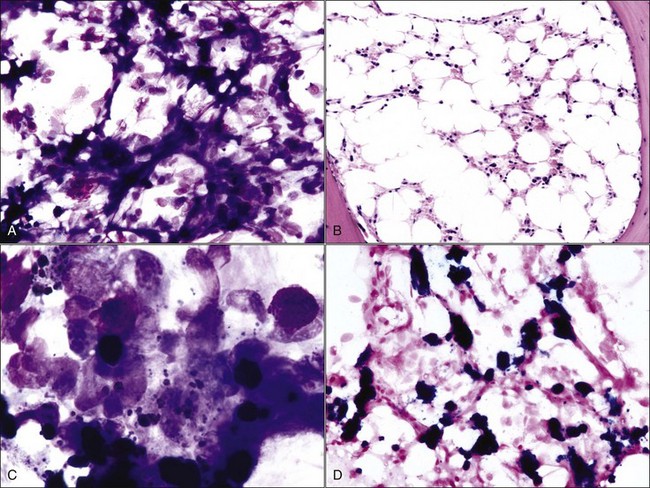
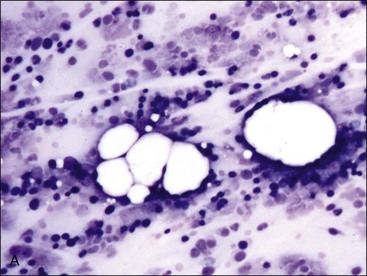
Gelatinous transformation of bone marrow (mucoid degeneration, serous atrophy of fat) involves morphologic changes in bone marrow that combine the loss of hematopoietic cells and the atrophy of fat with the deposition of gelatinous substances in the marrow spaces. Aspirated bone marrow has a mucoid consistency, resulting from the extracellular matrix material present. This material appears as a pink background in bone marrow aspirate smears stained with routine blood stains (Fig. 9-8, A) and in histologic sections stained with H&E (Fig. 9-8, B). Although the composition may vary somewhat, the material is composed of acid mucopolysaccharides, mainly hyaluronic acid, which stain strongly with Alcian blue at pH 2.5 (Fig. 9-8, C). The Alcian blue-positive staining is lost when samples are pretreated with bovine testicular hyaluronidase.40 Routine fixation in 10% neutral-buffered formalin is not ideal for staining acid mucopolysaccharides. Material is better preserved for optimal staining by fixation in 10% acid formalin with 70% alcohol.28 Edema may resemble gelatinous transformation in H&E-stained sections, but edematous fluid does not stain with Alcian blue.40
Gelatinous transformation of bone marrow in humans is generally associated with severe malnutrition accompanying disorders such as neoplasia, alcoholism, anorexia nervosa, infectious diseases (including acquired immunodeficiency syndrome [AIDS]), maldigestion, chronic heart failure, and metabolic disorders. Long bones are more likely to exhibit gelatinous transformation than flat bones.482 Widespread involvement may result in anemia, leukopenia, and less often thrombocytopenia.28,482
Gelatinous transformation of bone marrow has also been recognized in cachexic cats suffering from chronic diseases (including chronic renal disease and oral or gastric ulcers) and chronic anorexia.513 Bone marrow from cats with gelatinous transformation may still contain fat (see Fig. 9-8). Other reports of gelatinous transformation of bone marrow in animals include starvation in reindeer, 218 fluoride intoxication in calves,301 diet restriction in male Gottingen minipigs,41 and an emaciated miniature horse.28
Myelofibrosis
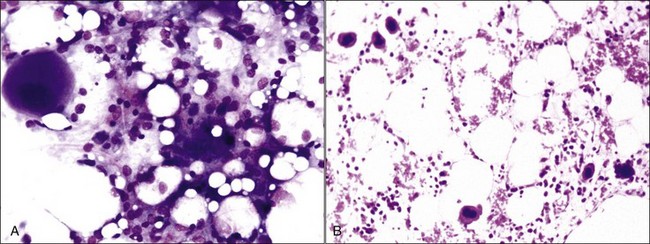
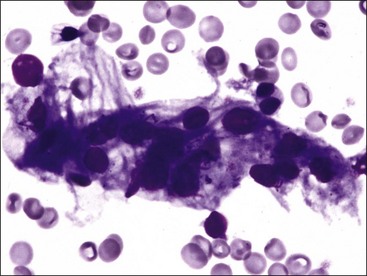
Myelofibrosis is suspected when repeated attempts at marrow aspiration are unsuccessful or a poor-quality aspirate is obtained that contains some spindle-shaped cells (Fig. 9-9). A definitive diagnosis can be made only by examining histologic sections of bone marrow.
Fibrous tissue consists of variable amounts of actively proliferating fibroblasts, reticulin fibers, and dense collagenous connective tissue.500 The term myelofibrosis is used when there is an apparent excess of reticulin/collagen fibers in bone marrow that is produced by activated and/or proliferating marrow reticular cells.488 Low levels of myelofibrosis may be definitively recognized only by using special stains (Fig. 9-10, A-C).
Type I collagen forms thick fibrils, while type III collagen forms thin fibrils. Reticulin fibers are visible as argyrophilic fibers in histologic sections of tissues stained using silver impregnating methods, such as Gomori stain (see Fig. 9-10, B).37 Reticulin fibers are primarily composed of individual type III collagen fibrils or small bundles of type III collagen fibrils that surround a core of type I fibrils. These fibrils are imbedded in a matrix of glycoproteins and glycosaminoglycans; silver stains appear to bind to this matrix rather than the collagen fibrils themselves.247 When present in normal marrow, reticulin fibers are located primarily in perivascular and peritrabecular areas.488 Larger collagen fibers composed primarily of type I collagen with less interfibrillar material are stained using a trichrome stain such as Masson trichrome (see Fig. 9-10, C). Few or no collagen fibers are visible using trichrome stains in normal bone marrow.247,488 The term reticulin fibrosis is used when argyrophilic fibers are increased in number and size; and the term collagen fibrosis is used when trichrome-positive fibers are present. Some fibrotic marrows also have increased vascularization, which results in increased amounts of type IV collagen in basement membranes.37 Reticulin fibrosis may be present without collagen fibrosis, but collagen fibrosis is almost never revealed by trichrome staining without increased reticulin.247
When myelofibrosis is extensive, it is recognized in sections stained with H&E (Fig. 9-11, A,B; Fig. 9-12, A,B). In these instances, marrow sections contain little or no fat. Hematopoietic cells may be present in linear arrangements, separated by palely eosinophilic extracellular collagenous material (see Fig. 9-11, B). Areas of marked myelofibrosis consist of fibroblasts and extracellular matrix, with no remaining hematopoietic cells (see Fig. 9-12). Increased hemosiderin is often present in bone marrow samples with prominent myelofibrosis (see Fig. 9-11, B; Fig. 9-12).37,488
Primary myelofibrosis (previously known as myelofibrosis with myeloid metaplasia or agnogenic myeloid metaplasia) in humans is a clonal myeloproliferative neoplasm. It is characterized by a proliferation of megakaryocytes and granulocytes, myelofibrosis, extramedullary hematopoiesis (especially in the spleen), and anemia with increased nucleated erythrocytes and immature neutrophils in blood (called leukoerythroblastic anemia in human hematology).454 A similar syndrome has not been described in animals, although myelofibrosis may be present in animals with various myeloid neoplasms.
Myelofibrosis appears to be a sequela to marrow injury, including necrosis, vascular damage, inflammation, and neoplasia.513 It is postulated that these disorders result in the direct or indirect production of cytokines capable of stimulating fibroblasts.388 Transforming growth factor-β (TGF-β), which is produced by a number of cell types including megakaryocytes and platelets, may be the most important factor in this regard.247
Prominent myelofibrosis has been documented in animals with marrow necrosis,519 myeloproliferative neoplasms,37,52,64,167 lymphoproliferative neoplasms,519 non-marrow-origin neoplasia,519 drug treatment (phenobarbital, phenylbutazone, and colchicine),515 nonregenerative IMHA in dogs and cats,447,513 and dogs with inherited pyruvate kinase deficiency.170,416 It has also been described as resulting from unknown causes.13,190,388,488,519
Marked myelofibrosis has been reported as a congenital (presumably inherited) disorder in young pygmy goats that also had megakaryocytic hyperplasia and dysplasia.62 Myelofibrosis has been described in a family of poodles with laboratory and clinical findings similar to pyruvate kinase deficiency,379 but definitive studies were not performed to eliminate the possibility that these animals had pyruvate kinase deficiency as well. It has been proposed that marrow fibrosis, like cirrhosis, occurs in response to damage caused by iron overload in pyruvate kinase-deficient dogs.551 However, factors associated with marked erythropoiesis may contribute to the development of myelofibrosis. Extremely high pharmacologic doses of recombinant human EPO elicited both marked erythropoiesis and myelofibrosis in experimental dogs.19
With the exception of dogs with inherited hemolytic anemias, animals with myelofibrosis typically have nonregenerative anemia. Blood leukocyte counts and platelet counts are often normal or increased in idiopathic cases of myelofibrosis, but they may be decreased, especially when collagen fibrosis is extensive.190,247 Multiple cytopenias are more likely to occur in animals with concomitant myeloid neoplasms.
Generalized Osteosclerosis/Hyperostosis
Osteosclerosis refers to a thickening of trabecular (spongy) bone, and hyperostosis refers to a widening of cortical (compact) bone from appositional growth of osseous tissue at endosteal and/or periosteal surfaces. Osteopetrosis is a form of osteosclerosis resulting from decreased bone resorption secondary to decreased numbers and/or abnormal function of osteoclasts.315,536 As a result of osteosclerosis and sometimes hyperostosis, the space available for hematopoiesis decreases. The remaining marrow space may appear hypocellular or exhibit fibrosis. Anemia occurs more often than thrombocytopenia or leukopenia. A variety of inherited, metabolic, inflammatory, and neoplastic disorders have been reported to cause generalized osteosclerosis in humans.536
Osteopetrosis has been described in dogs, cats, and horses with mild to severe nonregenerative anemia.31,241,262,341,344 Thrombocytopenia and neutropenia are less likely to be present.262,344 Osteopetrosis, anemia, thrombocytopenia, and marrow necrosis have been reported in beef calves naturally infected with BVD virus.414 Osteopetrosis occurs as an inherited disorder in Angus calves, which are typically aborted late in gestation.265 A defect in the gene that produces the SLC4A2 osteoclast anion exchanger prevents bone resorption due to the lack of acidification of the resorption lacunae.315
Osteosclerosis and myelofibrosis have been described in a dog with erythroid hypoplasia.105 Osteosclerosis and nonregenerative anemia have been reported in cats infected with FeLV, although it was suggested that these disorders occurred independently.192 Osteosclerosis and myelofibrosis occur in dogs with erythrocyte pyruvate kinase deficiency and in poodles with clinical and laboratory findings similar to those in documented cases of pyruvate kinase deficiency.372,379,416 The anemia in dogs with pyruvate kinase deficiency is regenerative, although the magnitude of the reticulocyte count may be lower as marrow pathology becomes more severe.372,416
Abnormalities of The Erythroid Series
Erythroid Hyperplasia
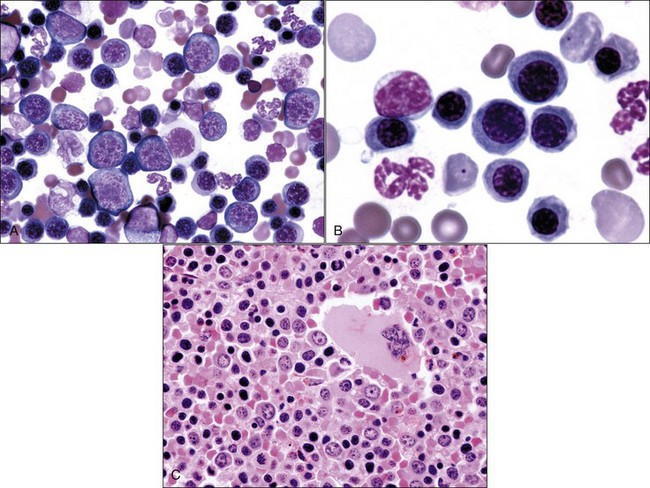
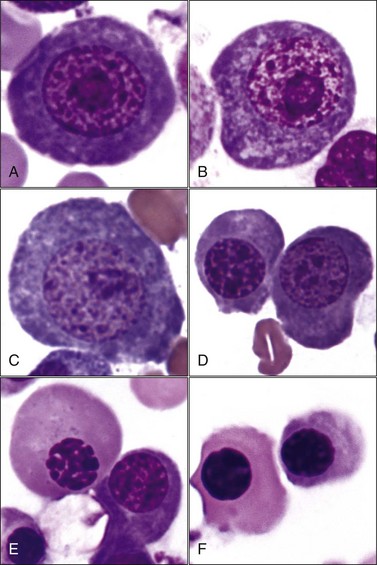
Erythroid hyperplasia is reported when the bone marrow cellularity is normal or increased, the absolute neutrophil count is normal or increased, and the myeloid : erythroid (M : E) ratio is low (Fig. 9-13, A-C). If the marrow is hypocellular and/or the absolute neutrophil count is low, a low M : E ratio indicates that granulocytic hypoplasia is present.
Approximately 4 days are required from the time an experimental animal is made anemic by phlebotomy for a peak reticulocyte response to occur in blood because this is the time required for reticulocytes to be produced following stimulation of erythroid progenitor cells by EPO.7,51,435 Early erythroid precursors can increase in bone marrow within 12 hours after EPO stimulation,476 but several days are probably required after hemorrhage or hemolysis has occurred before erythroid hyperplasia is prominent enough to result in a low M : E ratio. Bone marrow examination is generally not needed in anemic animals with an absolute reticulocytosis unless other cytopenias are also present.
Horses rarely release reticulocytes from the bone marrow even when an increased production of erythrocytes occurs. Consequently, bone marrow evaluation is often needed to determine whether an appropriate response to anemia is present in a horse. If the marrow cellularity is normal or increased and the neutrophil count is normal or increased, an M : E ratio below 0.5 suggests that a regenerative response to anemia is present (see Fig. 9-13).404
Erythroid hyperplasia may be effective (increasing hematocrit and/or reticulocytosis) or ineffective. Effective erythroid hyperplasia occurs in response to hemolytic or blood-loss anemia. It also occurs in response to primary or secondary erythrocytosis (polycythemia),151,175,231,494 although the M : E ratio is often within the reference range.306 Rubriblasts and prorubricytes are usually increased slightly in animals with effective erythroid hyperplasia; however, the predominant nucleated erythroid cells remain rubricytes and metarubricytes.189 Many polychromatophilic erythrocytes (reticulocytes) should be present in bone marrow aspirates when the erythroid hyperplasia is effective (see Fig. 9-13, B). A reticulocyte count may be done in the bone marrow aspirate to assist in this assessment. Bone marrow reticulocyte counts above 5% provide evidence for an effective regenerative response in horses.404
Ineffective erythroid hyperplasia may occur in severe iron deficiency,171 cobalamin deficiency in Border Collies (Fig. 9-14),324 folate deficiency in a cat (Fig. 9-15),330 certain myeloid neoplasms,107,208,308,319 congenital dyserythropoiesis,191,441 and in dogs and cats with nonregenerative IMHA (Fig. 9-16).216,447,512 The immune-mediated destruction of metarubricytes and reticulocytes, as well as other pathologic events that may be present (including vascular injury, inflammation, macrophage activation, myelodysplasia, myelofibrosis), apparently results in ineffective erythropoiesis.447,512
Selective Erythroid Hypoplasia or Aplasia
Erythroid hypoplasia is reported when the bone marrow cellularity is normal or decreased, the absolute neutrophil count is normal or decreased, and the M : E ratio is high (Fig. 9-17). When the M : E ratio exceeds 75 : 1 and morphologic abnormalities are not present in other cell lines, the terms selective erythroid aplasia or pure red cell aplasia are used.512 If the marrow is hypercellular and/or the absolute neutrophil count is high, a high M : E ratio indicates that granulocytic hyperplasia is present.
Selective erythroid aplasia occurs as either a congenital or acquired disorder in humans. Congenital erythroid aplasia in humans (Diamond-Blackfan anemia) appears to represent a heterogeneous group of genetic disorders.73,271 Approximately 40% of cases are associated with other congenital defects, especially malformations of the head and upper limbs. Acquired erythroid aplasia in humans may occur in association with disorders including B-19 parvovirus infection, proliferative disorders involving large granular lymphocytes, anti-EPO antibodies (primarily from treatment with recombinant EPO), thymomas, and myelodysplastic syndrome (MDS). Erythroid aplasia has also been reported in humans in association with the administration of many drugs as well as with autoimmune diseases, various hematopoietic neoplasms, solid tumors, infections, vascular diseases, pregnancy, and severe renal failure.408
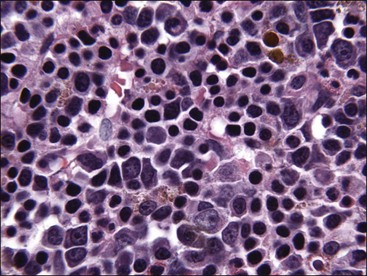
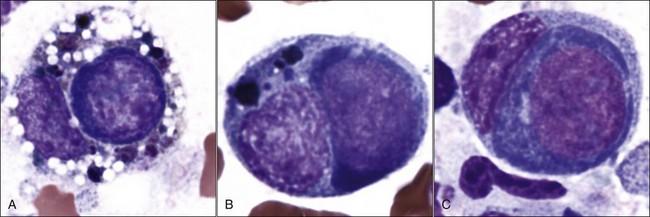
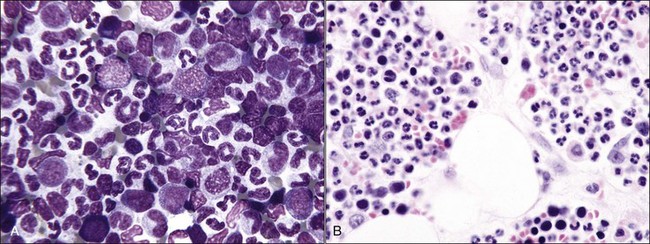
Acquired selective erythroid hypoplasia or selective erythroid aplasia occurs in dogs and cats when an immune response is directed against early erythroid precursor cells (Fig. 9-17, A,B).447,512 This response may develop through antibody- or cell-mediated mechanisms that completely inhibit erythroid production or result in a maturation arrest at various stages of erythroid maturation (Fig. 9-18). Phagocytosis of early erythroid precursor cells may sometimes be recognized in selective erythroid hypoplasia (Fig. 9-19, A-C). An immune-mediated attack might be targeted against a maturation-associated antigen, resulting only in the destruction of erythroid precursors in the marrow, or at a common antigen on precursors and mature erythrocytes, which would result in the destruction of precursors in the bone marrow and concurrent erythrocyte destruction in the circulation.447 The classification of these disorders as being immune-mediated is largely based on positive responses to immunotherapy, but some animals have positive Coombs and/or antinuclear antibody (ANA) tests. In addition, antibodies in the serum of some dogs with selective erythroid aplasia have been reported to inhibit erythropoiesis in bone marrow cultures.499 Finally, increased numbers of small lymphocytes in the bone marrow of cats (and some dogs) with selective erythroid hypoplasia and selective erythroid aplasia (Fig. 9-20) suggest that cell-mediated immune mechanisms may be important in the pathogenesis of these disorders.446,447,512
Erythroid hypoplasia or aplasia is a common finding in animals with MDS and acute myeloid leukemia (AML) that have prominent proliferative abnormalities in granulocytic and/or megakaryocytic cell lines.436,516 Erythroid hypoplasia is reported to be a rare sequela to vaccination against parvovirus in dogs.100 High doses of chloramphenicol cause reversible erythroid hypoplasia in some dogs495 and erythroid aplasia in cats (Fig. 9-21).497 Erythroid aplasia, together with megakaryocytic aplasia and neutrophilic hyperplasia, is seen in early estrogen toxicity in dogs.423,501 A dog has been reported to have congenital erythroid aplasia based on histopathologic examination of bone marrow at necropsy, but the M : E ratio was normal when aspirate smears were examined several days prior to euthanasia.197 Transient erythroid hypoplasia apparently occurs at regular intervals in gray Collie dogs with inherited cyclic hematopoiesis, but it does not cause anemia because it is of short duration and followed by a period of erythroid hyperplasia.1,251,413
Selective erythroid hypoplasia or aplasia occurs in cats infected with FeLV subgroup C but not in those infected only with subgroups A or B (Fig. 9-22, A,B).398 The cell surface receptor for FeLV-C is called FLVCR. This receptor has been demonstrated to be a heme exporter.376 Free heme is toxic to cells, and it appears that the binding of FeLV-C to FLVCR on rubriblasts inhibits heme export from these cells, resulting in their destruction.226 This exporter can also export protoporphyrin IX and coproporphyrin and appears to be important in heme recycling by macrophages. The plasma protein hemopexin facilitates the export of heme from tissues and the transport of heme to the liver.546
Marked erythroid hypoplasia has been reported in dogs, cats, and horses given recombinant human EPO.90,364,540 Antibodies made against this human recombinant glycoprotein apparently cross-react with the animals’ endogenous EPO.
Erythroid production is reduced in chronic renal disease189 and endocrine deficiencies (hypopituitarism, hypoadrenocorticism, hypothyroidism, and hypoandrogenism) but is not usually pronounced enough to result in an M : E ratio in the marrow that is increased above the reference interval.
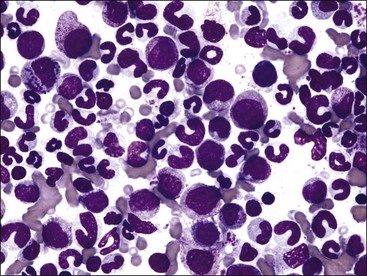
A mild to moderate nonregenerative anemia often accompanies chronic inflammatory and neoplastic disorders. The cause of this anemia of inflammatory disease (anemia of chronic disease) is multifactorial and only partially understood. Abnormalities that can contribute to the anemia include the production of inflammatory mediators that directly or indirectly inhibit erythropoiesis, decreased serum iron, shortened erythrocyte life spans, and blunted EPO response to the anemia.171 The M : E ratio is typically high in clinical cases of the anemia of inflammatory disease in dogs (Fig. 9-23, A,B),189 not only because of deficient erythropoiesis but also because of concomitant granulocytic hyperplasia.11
Dyserythropoiesis
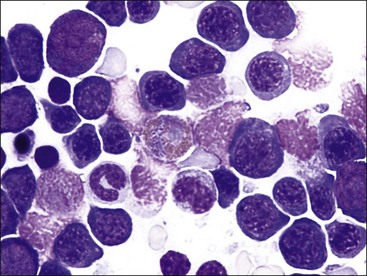
Megaloblastic erythroid cells are larger than normal, with a more stranded arrangement of chromatin and abundant parachromatin, giving a pronounced light and dark pattern to the nucleus (Fig. 9-24, A-E). The cytoplasm is generally abundant and hemoglobin synthesis may be present at earlier stages of development than typically seen (e.g., nuclear and cytoplasmic asynchrony). Rubricytes and metarubricytes may be macrocytic without prominent nuclear abnormalities (Fig. 9-24, F). These morphologic abnormalities are most often seen in animals with myeloid neoplasms.* Megaloblastic erythropoiesis occurs most commonly in ill cats with FeLV infections, but it has also been reported in cats with feline immunodeficiency virus (FIV) infections.426 Megaloblastic erythroid cells have been reported in the marrow of cats with natural and experimentally induced folate deficiency (see Fig. 9-15).330,464 Finally, some miniature and toy poodles exhibit a nonanemic macrocytosis, metarubricytosis, and/or erythrocytes with multiple Howell-Jolly bodies and variable megaloblastic abnormalities in the bone marrow. Serum folate and B12 values are normal in this hereditary poodle macrocytosis.66,410,514
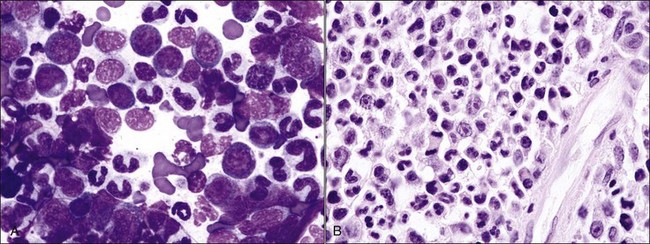
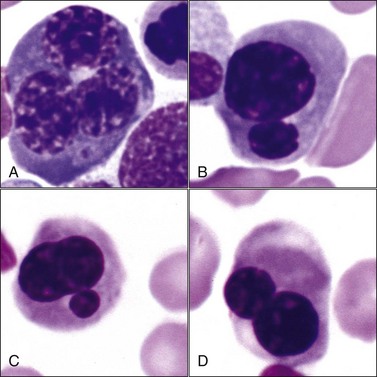
Multinucleated erythroid cells (Fig. 9-25, A) have been reported in animals with myeloid neoplasms209,309,319,530 and in those with acquired and congenital dyserythropoiesis.275,441,531 Nuclear lobulations, pyknosis, and/or fragmentation may occur in animals with myeloid neoplasms,88,426 acquired and congenital dyserythropoiesis,185,191,441,531 and following treatment with drugs that interfere with DNA synthesis, including antimetabolites (e.g., azathioprine, hydroxyurea, cytosine arabinoside), alkylating agents (e.g., cyclophosphamide), folate antagonists (e.g., methotrexate), and plant alkaloids (e.g., vincristine) (Fig. 9-25, B-D).5,38 Internuclear chromatin bridging has been reported in cattle with congenital dyserythropoiesis and in hereditary poodle macrocytosis.441,514
Maturational arrests at various stages of erythroid development, with a resultant lack of polychromatophilic erythrocytes, may occur in acquired myeloid neoplasms,39 in congenital dyserythropoiesis,441 and in some drug-induced disorders (e.g., cephalosporin antibiotics).97 It is a common finding in nonregenerative IMHA, especially in dogs (see Fig. 9-16).216,443,447 A nonregenerative immune-mediated anemia with erythroid maturation arrest has also been reported in a ferret.291 Asynchrony of nuclear and cytoplasmic maturation, in which hemoglobinization precedes nuclear maturation, may occur in acquired myeloid neoplasms as well as in congenital dyserythropoiesis.207,441
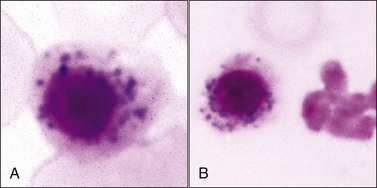
Iron-positive basophilic stippling has been reported in rubricytes and metarubricytes (nucleated siderocytes) from animals with myeloid neoplasms,39,88,185,528,530 dogs with idiopathic dyserythropoiesis (including inflammatory disorders),67,172,506 and the use of drugs/chemicals, including chloramphenicol (Fig. 9-26, A,B), hydroxyzine, lead, zinc, and an oxazolidinone antibiotic.169,174,276,365
Abnormalities of The Granulocytic Series
Neutrophilic Hyperplasia
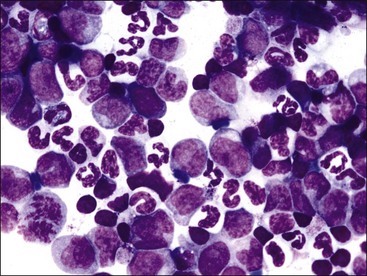
Neutrophilic hyperplasia may be effective or ineffective. Effective neutrophilic hyperplasia results in a peripheral neutrophilia, with or without a left shift. It occurs in response to various hematopoietic growth factors, with granulocyte-colony stimulating factor (G-CSF) being most important.11 Two or more days are required from the time of growth factor stimulation until neutrophilic hyperplasia is prominent enough to increase the M : E ratio outside of the reference interval.11,152
Neutrophilic hyperplasia occurs most frequently in response to bacterial infections, but it may also occur in response to immune-mediated inflammatory disorders, necrosis, chemical and drug toxicities, and malignancy (Fig. 9-27, A,B).123,207 The natural release or injection of recombinant G-CSF, granulocyte/macrophage-colony stimulating factor (GM-CSF), and interleukin (IL)-3 result in peripheral neutrophilia and neutrophilic hyperplasia in the bone marrow.92,338,348,477,553 Extreme neutrophilic hyperplasia in bone marrow and peripheral neutrophilia have been reported as a paraneoplastic syndrome in dogs and cats with tumors that produce hematopoietic growth factors.102,253,421,465
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree