Chapter 11 Disinfectants, Antiseptics, and Related Biocides
Disinfectants, antiseptics, and biocides are expected to play an even more important role in the future in controlling microbes in both the veterinary patient and hospital.1 When used properly, disinfectants, antiseptics, and biocides contribute to both the prevention and the treatment of disease. Despite the fact that there are as many as 300 biocidal products available, about 14 of these are in more than 90% of the registered products in the United States.2 The veterinary clinician need be familiar with relatively few biocidal products to make an informed decision regarding their selection and use.
Definitions
Definitions of appropriate terms, characteristics of disinfectants and antiseptics by chemical type, factors affecting disinfection and antisepsis, and disinfection and antiseptic practices germane to veterinary practice are reviewed in this chapter (Box 11-1). It is hoped that through greater understanding of the properties of disinfectants and antiseptics, veterinary clinicians will use them appropriately.
Box 11-1 Disinfection and Antisepsis Definitions
(From Block SS: Disinfection, sterilization, and preservation, ed 5, Philadelphia, 2001, Lippincott Williams & Wilkins; and Greene CE: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Saunders)
The distinction between disinfectants and antiseptics is not always clear (Table 11-1). Antiseptics are usually the weakest and least toxic of the surface antimicrobials.3 Antiseptics may be used on intact skin or mucous membranes before a surgical procedure or in the treatment of open wounds. Regardless of their use, antiseptics should exert a sustained effect against microorganisms without causing tissue damage.4 Although some specific biocides may be used as both disinfectants and antiseptics (e.g., alcohols and iodines), it is not generally recommended that an antiseptic be used for the purpose of disinfection, and vice versa.
Table 11-1 Categorization of Biocides: Disinfectants, Antiseptics, or Both
| Biocides Most Appropriately Used as Disinfectants | Biocides Most Appropriately Used as Antiseptics | Biocides Effective as Both Disinfectants and Antiseptics |
|---|---|---|
| Aldehyde compounds (formaldehyde and glutaraldehyde) | Chlorhexidine | Alcohols |
| Chlorine and chlorine compounds | Dilute sodium hypochlorite solution (Dakin’s solution) | Chloroxylenols |
| Ethylene oxide | EDTA | Iodines |
| Hydrogen peroxide | Iodophors | |
| Phenols | ||
| Quaternary ammonium compounds |
Characteristics of Disinfectants and Antiseptics by Chemical Type
The characteristics of the following 10 types of disinfectants, antiseptics, and biocides are presented: alcohols, aldehyde compounds (formaldehyde and glutaraldehyde), chlorhexidine, chlorine and chlorine compounds, ethylenediaminetetraacetate (EDTA), ethylene oxide, iodine and iodine compounds, peroxygen compounds (including hydrogen peroxide), phenols (including bisphenols and halophenols), and quaternary ammonium compounds. Mechanism of action (presumed or established), classification as to level of biocidal activity, commonly available preparations, efficacy, and uses are presented for each chemical type (Figure 11-1).
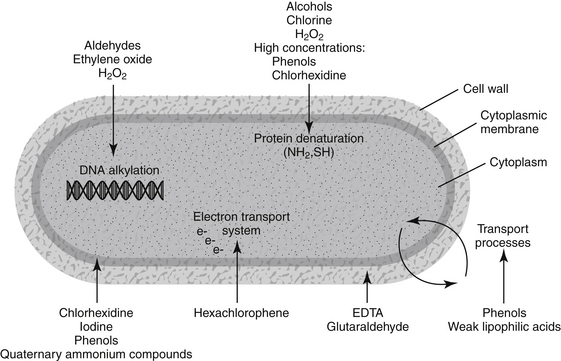
Figure 11-1 Diagram showing targets within the microbe for selected disinfectants, antiseptics, and biocides.
Alcohols
Alcohols possess the following features desirable for a disinfectant: bactericidal action against vegetative forms, relative inexpensivness, ease of availability, and relative nontoxicity when used topically.5 Alcohols are used alone or in combination with phenols, chlorhexidine, iodines, and quaternary ammonium compounds.6 Alcohols appear to exhibit their antimicrobial effect by denaturing proteins. Lysis of some microorganisms may occur, although the bacteriostatic action of alcohols is due to the inhibition of cell metabolites. Some water is required for alcohols to be most effective. Alcohols are considered to have intermediate-level biocidal activity. Two forms of alcohol are used most commonly: ethyl alcohol and isopropyl alcohol. Ethyl alcohol has enhanced virucidal properties and reduced toxicity compared with isopropyl alcohol, which has slightly greater bactericidal action.
When used alone, alcohols are more effective antiseptics than disinfectants. Alcohols are not good cleaning agents and are not recommended in the presence of physical dirt.7 Alcohols are widely used for both hard-surface disinfections and skin antisepsis.8 In appropriate concentrations, alcohols provide the most rapid and greatest reduction in microbial counts on intact skin. Alcohols should not be used in open wounds. Alcohols should be allowed to evaporate thoroughly from the skin to be fully effective and to decrease irritation.7 Because of their inability to destroy bacterial spores, alcohols are not recommended for disinfection of surgical instruments.
Aldehyde Compounds (Formaldehyde and Glutaraldehyde)
Formaldehyde (3% to 8% solutions) exhibits intermediate-level to high-level disinfection. It is sporicidal, with its mechanism of action being the ability to combine with protein, RNA, and DNA in the spore.9 Formaldehyde does not penetrate well, and its fumes are irritating.6 It is used infrequently as a disinfectant in veterinary hospitals.
Glutaraldehyde (1,5-pentanedial) has been used as a chemosterilizing agent for approximately 40 years.10,11 It displays potent bactericidal, fungicidal, virucidal, mycobactericidal, and sporicidal activity.11–13 Glutaraldehyde acts on proteins by denaturation and on nucleic acids by alkylation.14 It is classified as an intermediate-level to high-level biocide.
Factors that influence the activity of glutaraldehyde include time of contact, temperature, concentration, pH, and the presence of soiling material.12 Glutaraldehyde shows a very marked, temperature-dependent activity.12 The pH affects the stability and biocidal activity of glutaraldehyde. Glutaraldehyde is more stable at acid pH, but it is more active at alkaline pH (around 8 to 8.5). As pH increases, the number of reactive sites to which glutaraldehyde binds is increased, thus enhancing its lethal effect.12 At alkaline pH, glutaraldehyde penetrates more extensively into the spore, where it fixes the cortex.11 The negative effect of organic matter is more apparent when lower concentrations of glutaraldehyde are used. Alkaline glutaraldehyde (2%) takes longer to be effective in the presence of organic matter.6 Glutaraldehyde has a dual role against bacterial biofilms (a characteristic of some bacteria that makes them more resistant to disinfection): an ability to penetrate the biofilm and inhibit microbial cells protected by the film and an acceleration of the detachment rate of bacteria from the biofilm.12
Disinfectants containing 2% glutaraldehyde are considered high-level disinfectants, with recommended contact times of 10 to 30 minutes. Exposure times of 6 to 10 hours frequently result in sterilization. Glutaraldehyde is used widely as a disinfectant for heat-labile equipment (e.g., endoscopes). Glutaraldehyde disinfectants are not as noxious, irritating to the skin, or corrosive as formaldehyde; however, precautions should be taken with their use. Gloves, safety goggles, and proper ventilation are recommended to minimize risks to those disinfecting the equipment, and glutaraldehyde-disinfected equipment should be thoroughly rinsed with sterile water before use to reduce risk to the patient.11 Stabilized glutaraldehyde solutions are safe and effective preoperative skin antiseptics for elective clean-contaminated surgical procedures in dogs.15
Chlorhexidine
Chlorhexidine is a cationic bisbiguanide, not related to hexachlorophene, that was first synthesized in 1950.16 It is available as a solution and as a scrubbing agent. Chlorhexidine solution is used principally as a topical antiseptic on skin wounds and mucous membranes, but it is also used as a pharmaceutical preservative. Chlorhexidine scrub is used to preoperatively prepare the surgeon and patient. Chlorhexidine exhibits a broad spectrum of antibacterial activity, strong binding to the skin, ability to adsorb to negatively charged surfaces in the mouth (e.g., tooth and oral mucosa), persistence, low toxicity, and a minimal negative effect on activity by blood or other organic material.7 Chlorhexidine has its major antibacterial action by interference with the function of cellular membranes, with the primary site of action being the cytoplasmic membrane.8,13,14,16,17 Rupture of the cytoplasmic membrane of the microbe occurs without lysis of the cell wall. Chlorhexidine has low-level to intermediate-level biocidal activity.
Chlorhexidine has a very rapid bactericidal effect as well as persistence of action.14 It has limited fungicidal and virucidal properties.6 Chlorhexidine is more effective against gram-positive than gram-negative bacteria and exhibits a bacteriostatic effect against some bacteria.6,7 Acquired resistance to chlorhexidine has been observed, notably in staphylococci.8,18 The optimum range of pH for activity of chlorhexidine is 5.5 to 7. Chlorhexidine is incompatible with anionic detergents and inorganic anionic compounds; 6 thus standing soap lather should be removed by rinsing before chlorhexidine solution is applied to the skin. Chlorhexidine forms a precipitate when diluted with electrolyte solutions, but this precipitate does not affect antimicrobial activity.19
Chlorhexidine is available as an alcoholic solution (scrub) that is used in the preoperative skin preparation of the surgeon and patient and as an aqueous solution. Two preparations that are used commonly in veterinary hospitals are chlorhexidine gluconate (scrub) and chlorhexidine diacetate or gluconate (solution). There are few reports of adverse reactions with chlorhexidine. Chlorhexidine scrub should be used only on intact skin, never in wounds. Negligible absorption from the alimentary tract occurs, and the incidences of skin irritation and hypersensitivity are low. Chlorhexidine is ototoxic when placed in the middle ear cavity, and its use on the brain or meninges is contraindicated. In general, chlorhexidine solution (0.05%) is an effective and well-tolerated wound antiseptic in veterinary patients.19,20
KEY POINT 11-3
Chlorhexidine solution (0.05%) is generally an effective and well-tolerated wound antiseptic.
Chlorine and Chlorine Compounds
Chlorine disinfectants are readily available, inexpensive, have a broad antimicrobial spectrum, and present minimal environmental hazards.3 Mechanism of action appears to be through oxidation of peptide links and denaturation of proteins.14 Intracellular accumulation results in inhibition of essential enzyme systems.13 Chlorine compounds are classified as intermediate-level disinfectants. Two factors that affect the biocidal activity of chlorine are pH and the presence of organic material. The greatest influence on the antimicrobial activity of chlorine in solution appears to be pH. With decreasing pH there is increasing biocidal activity. This increased activity at lower pH is due to a higher concentration of undissociated hypochlorous acid, which has a greater bactericidal action than the dissociated form. Organic matter consumes available chlorine and reduces its antibacterial efficacy.21 This negative effect is particularly evident in solutions with low levels of chlorine. Small additions of iodine or bromine to chlorine solutions greatly enhance their bactericidal activity. 21
Sodium hypochlorite appears to be the chlorine compound most frequently used as a disinfectant in veterinary hospitals. It is an effective virucidal agent.22 Sodium hypochlorite was first introduced as an antiseptic in 1915 as Dakin’s solution (0.4% available chlorine).23 Although still used as a wound antiseptic, sodium hypochlorite is used more frequently as a disinfectant in veterinary hospitals as a 0.16% solution (1:32 dilution of 5.25% stock solution) of liquid bleach.
EDTA
EDTA, especially in combination with Tris buffer ([hydroxymethyl]aminomethane), has been shown to have antibacterial properties, particularly against certain gram-negative bacteria. Tris-EDTA acts by increasing cell wall and membrane permeability through chelation of divalent cations and by slowing degradation of ribosomes.13,24,25 The clinical use of Tris-EDTA has been limited largely to four major pathogens: Pseudomonas aeruginosa, Proteus vulgaris, Escherichia coli, and Staphylococcus aureus.24 Tris-EDTA decreases the minimal inhibitory concentration for these bacteria when selected antimicrobials are added in vitro.26 It also potentiates the antimicrobials effects of chlorhexidine diacetate in lavage solutions.27
Tris-EDTA solution is inexpensive and readily available. It has been used as an irrigant in combination with antimicrobials in the treatment of otitis externa, bacterial rhinitis, and multiple fistulae in dogs.24 Formulations of other biocidal agents (e.g., chloroxylenol) may contain EDTA. Such preparations have enhanced efficacy against P. aeruginosa organisms. 28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


