Mats H.T. Troedsson, Bruce W. Christensen, Consulting Editors ▪ Female Reproductive Disorders Bruce W. Christensen, Bret R. McNabb, Mats H.T. Troedsson, Elizabeth M. Woodward The mare is a seasonally polyestrous animal, breeding during seasons of long day length. Annual breeding and nonbreeding seasons are divided by fall and spring transitional periods, which are characterized by erratic reproductive behavior and irregular estrous cycles. During the breeding season, mares ovulate every 21 days (range 19 to 22 days).1 Estrus (5 to 7 days, but variable) is characterized by the presence of an ovarian follicle, serum progesterone level of less than 1 ng/mL, and sexual receptivity. During estrus the cervix is palpably relaxed and the uterus is edematous. One or two follicular waves occur per cycle, and preovulatory follicles are 45 to 60 mm in diameter, often with a cone-shaped appearance on ultrasonography.2 Ovulation occurs 24 to 48 hours before the end of estrus and may be accompanied by ovarian sensitivity.1 The ruptured follicle is replaced by a corpus luteum (CL). Diestrus (luteal phase) is predictable in length because regression of the CL, caused by release of endometrial prostaglandin F2α (PGF2α), occurs 14 to 15 days after ovulation.3 During diestrus and early pregnancy the cervix is tight and the uterus has increased tone. Diestrous ovulations occur and may be fertile.4 First postpartum estrus (“foal heat”) begins in the week after foaling, and ovulation occurs in most mares 7 to 15 days postpartum. Cows are polyestrous, but seasonal differences in fertility may be caused by climate. The estrous cycle averages 21 days (range 17 to 25 days), and the duration of estrus averages 12 to 16 hours (range 6 to 24 hours). Cows are unique among domestic animals in that they ovulate spontaneously after the end of estrus, 24 to 30 hours after the beginning of estrus.5 In the absence of a bull, estrus can be detected in cows by their homosexual (bisexual) activity. Cows that stand to be mounted by another cow are in estrus (standing heat). Secondary signs that may be helpful in detecting estrus include restlessness and increased activity, vulvar hyperemia and edema, and a clear mucous discharge. Errors in heat detection are a common cause of infertility on large dairy farms.6 The optimum time for insemination of cows is between 16 and 24 hours after the onset of estrus. Insemination of cows on the basis of standing to be mounted results in a higher pregnancy rate than if it is based on secondary signs of estrus.7 Well-managed dairy cows with uncomplicated periparturient experiences may ovulate approximately 20 to 25 days after calving, whereas beef cows with nursing calves usually do not ovulate until 40 or more days after calving. The presence of the calf appears to be responsible for the difference in return to cyclicity.8 Coarse-wooled breeds of ewes are seasonally polyestrous during the autumn and winter (short photoperiod) in temperate climates. Ovulation can be induced in seasonally anestrous ewes by artificial simulation of day length and temperatures characteristic of autumn (reduced photoperiod and reduced ambient temperature), but the long latency period required for response to manipulation of light and temperature makes the procedure impractical. Ewes of fine-wooled breeds may be polyestrous throughout the year if adequately nourished. The estrous cycle of ewes averages 17 days (range 14 to 19 days), and the duration of estrus averages 36 hours. Ovulation occurs spontaneously 24 hours after the onset of estrus. Ewes display few, if any, signs of estrus unless a male is present. The primary signs of estrus include seeking the ram and standing for mating. Secondary signs include restlessness and rapid tail switching; vulvar edema and discharge of clear cervical mucus may be observed occasionally. Lambing ordinarily occurs during the anestrous season; therefore ewes do not return to estrus until the next breeding season.5 Does in temperate climates are seasonally polyestrous from late summer until early spring (short photoperiod). Onset of the breeding season in yearling does can be advanced by exposing them to 19 hours of artificial light per day for 70 days beginning in mid to late winter. Termination of artificial light results in a relative decrease in day length and stimulation of estrus and ovulation.5 Alternatively, the breeding season may be hastened by exposing does to 14 to 18 hours of light per day for 3 months, followed by a reduction to 6 hours of light per day.7 The estrous cycle averages 21 days, and estrus lasts 18 to 36 hours. Ovulation occurs spontaneously 24 hours after the onset of estrus. An intact male or male pheromone is usually necessary for estrous detection. The primary signs of estrus are seeking the buck and standing for service. Secondary signs of estrus in does include rapid tail switching, restlessness, increased frequency of urination and vocalization, transient decrease in appetite and milk production, and edema and hyperemia of the vulva. As in sheep, parturition takes place during the anestrous season, and return to cyclicity in does is delayed until the next breeding season.5 South American camelids bred in North America are nonseasonal. In keeping with management decisions, they are often bred in a seasonal manner to avoid having newborn crias during the hottest or coldest months of the year. Some consider the South American camelids to be polyestrous, whereas others argue that they do not have a true estrous cycle.9 These discrepancies arise from the fact that camelids are induced ovulators. Cyclic ovarian activity (e.g., transition from estrus to diestrus) is caused by coital activity. Unbred female camelids essentially exhibit estrous behavior continually, with perhaps short, occasional, unpredictive intervals of 1 to 2 days of decreased receptivity. Follicular waves in alpacas and llamas last around 17 days. Small follicles (<3 mm) are always present on the surface of the ovaries. After recruitment, follicles grow to approximately 5 mm in size. Dominance is established when one of the follicles reaches the size of 6 mm, and the female will show signs of receptivity to the male as long as a dominant follicle is present. The sizes of preovulatory follicles are 9 to 13 mm and 8 to 12 mm in the llama and alpaca, respectively. Females maintain fertile, dominant follicles for an average of 8 days before undergoing regression. Usually the next follicular wave has already produced the next dominant follicle before the previous preovulatory follicle begins to regress. For this reason, the female camelid usually maintains estrous behavior continually until she is mated.9 Ovarian cysts are follicle-like ovarian structures that arise because of failure of ovulation of a dominant follicle.5,10 They are usually larger than 25 mm in diameter and persist in the absence of a CL for 10 days or more. Follicular cysts have thin walls and may be single, multiple, or multilocular structures on one or both ovaries. Partially luteinized cysts tend to be single, unilateral structures with thicker walls because of the presence of luteal tissue. The mechanism by which ovulation fails and cysts develop is not known. Failure of ovulation may result from inadequate release of gonadotropins or ovarian dysfunction. Increased stress of conditions such as retained placenta, metritis, and hypocalcemia around the time of calving and postpartum ketosis have been associated with an increased prevalence of cystic follicular degeneration (CFD), as has a hereditary predisposition.11 Negative energy balance in high-producing dairy cows, as well as prolonged suckling in beef cows, may inhibit lutenizing hormone release from the pituitary and subsequent failure of ovulation.12 Approximately 70% to 80% of cows affected by CFD are anestrus, whereas 20% to 30% display frequent or intense estrus (nymphomania). Cystic ovarian disease affects 10% to 30% of dairy cows. The condition is rare in commercial beef cows because of rigid culling for reproductive failure. The physical appearance of cows with CFD depends on the duration of the condition. No changes are apparent after a short time, but in long-standing cases relaxation of the pelvic ligaments may result in prominence of the tailhead and masculine characteristics such as a crested neck. The diagnosis of CFD is based on an accurate history and clinical examination. A history of constant or frequent estrus, short interestrous intervals, or anestrus may suggest CFD. Examination of the ovaries by palpation per rectum reveals the presence of enlarged fluid-filled structures raised above the surface of the ovary that greatly increase total ovarian size. Ovarian cysts are larger (>25 mm) than preovulatory follicles (15 to 25 mm). Differentiation between a single large cyst and several smaller cysts on the same ovary may require ultrasonographic examination, as does recognition of the presence of partially luteinized cysts (based on peripheral progesterone concentrations). Ovarian cysts appear to be dynamic structures; those that develop early in the postpartum period may regress without treatment, and a normal estrous cycle may follow, or another cystic structure may develop. During palpation of the ovaries, several normal structures may complicate the diagnosis of CFD. Normal preovulatory follicles may approach 25 mm in diameter and have palpable characteristics similar to those of small cysts. During the follicular phase of the estrous cycle, however, the uterus responds to palpation by becoming more turgid, whereas the uterus of a cow with CFD is typically flaccid and unresponsive. In neglected cases of CFD, mucometra may develop and must be differentiated from pregnancy. During the first 5 to 7 days of the estrous cycle, the developing CL may be smooth and soft and is commonly mistaken for an ovarian cyst. More mature CLs are solid and liver-like in consistency, often feature a palpable ovulatory papilla at the apex, and are more easily differentiated from ovarian cysts. However, 10% to 20% of mature CLs may lack an ovulatory papilla, making them more easily confused with ovarian cysts. Ultrasonography is generally more accurate in identifying subtle structural differences than transrectal palpation. Salpingitis, hydrosalpinx, oophoritis, ovarian abscesses, ovarian neoplasms, and cysts of the fimbria are other causes of enlargement of the ovary and surrounding structures that must be differentiated from ovarian cysts.13 Histories that may erroneously suggest CFD include apparently short interestrous intervals because of inaccurate detection of estrus.14 Oxytocin administered to stimulate milk letdown may result in short interestrous intervals and suggest CFD. The estrous cycle may be shortened by administering 100 IU of oxytocin per day on days 2 through 615 or on days 3 through 7 or 8.16 Heifers treated with 100 IU of oxytocin per day returned to estrus in an average of 12.9 days versus 20.3 days in untreated controls. Plasma progesterone concentrations are low in cows with follicular cysts. Partial luteinization may occur, and progesterone concentrations may increase over time but remain lower than those of cows with normal CLs. Estrogen concentrations in the plasma of cows with CFD are variable. The goal in treating CFD is to induce luteinization of the cyst and reestablish normal estrous cycles. Several methods have been recommended. Spontaneous recovery from CFD occurs in up to 60% of cows that develop CFD before the first ovulation after calving but in only approximately 20% of cases that develop after the first postpartum ovulation. Evaluation of therapeutic agents for CFD may be confounded by spontaneous recovery. Recommended doses of human chorionic gonadotropin (hCG) range from 5000 IU either intravenously (IV) or intramuscularly (IM) to 10,000 IU IM. Of cows treated with a single dose of hCG, 65% to 80% establish a normal estrous cycle within 3 to 4 weeks; a second or third dose may be required in cows that do not respond after 3 to 4 weeks or in cases in which nymphomania persists. Anaphylaxis after repeated treatments with a larger protein hormone such as hCG can occur. Antibodies to hCG may reduce the effectiveness of sequential treatments. Therapeutic response to follicular cysts, both endocrinologically and clinically, is essentially equivalent between hCG and gonadotropin-releasing hormone (GnRH). Currently the most common treatment for ovarian cysts, especially follicular cysts, is an injection of GnRH (100 µg IM). Cows responding to this treatment have an average interval to estrus of one estrous cycle or 18 to 24 days. The treatment to breeding interval can be shortened by administering GnRH at the time of diagnosis, followed by a luteolytic dose of prostaglandin 10 days to 2 weeks later. With this regimen it is not critical whether the cyst is follicular or luteal or even whether it is a misdiagnosed large, smooth CL with or without a fluid-filled central cavity. Most veterinarians agree that accurate differential diagnosis by rectal palpation among follicular cysts, luteal cysts, and some CLs can be a problem. Luteal-type cysts can be treated with the luteolytic activity of PGF. The advantage is the quicker return to estrus for those cows able to respond and the lower cost of PGF. Cysts that luteinize in response to GnRH regress at a time similar to that of normal CLs. Treatment with PGF may be used to reduce the interval from treatment with GnRH to estrus from 18 to 24 days to an average of 12 to 14 days by administering PGF at 9 days after GnRH. Most clinicians are only approximately 50% accurate in determining the degree of luteinization of cysts by palpation per rectum; therefore measurement of concentrations of progesterone in milk or of plasma from affected cows allows the selection of GnRH or hCG for treatment of follicular cysts and PGF for the treatment of luteinized cysts. Ultrasonography can also be used to make an accurate diagnosis.17 Thin-walled follicular cysts may be inadvertently ruptured during examination of the ovaries, and some practitioners may intentionally attempt cyst rupture. Recovery rates after manual rupture have rarely been studied in well-designed controlled experiments but are generally within the range reported for spontaneous recovery. Deliberate manual rupture of ovarian cysts is considered an obsolete form of treatment by some veterinary clinicians, but others routinely use the procedure—especially as an initial treatment for cysts found during the voluntary waiting period. Manual rupture of cysts may be followed by hemorrhage and adhesions between the ovary and surrounding structures. These complications appear to be much more common with use of digital pressure to enucleate CLs than with manual rupture of ovarian cysts. Cystic ovaries appear to be more common in goats than in sheep. In one study 12% of goats had cystic ovaries when examined at a slaughterhouse.18 The condition is often overdiagnosed by owners observing nymphomania. Treatment typically consists of administering exogenous ovulation-hastening drugs (hCG and GnRH).19 LH surge is noted usually within 2 hours of GnRH administration. PGF may be administered 10 days later to bring the doe into heat (55 hours after PGF). Hemorrhagic follicles are observed in nonbred llamas and usually regress within 22 days.20 Follicles larger than 12 mm are considered by some authors as pathologic cysts. These structures may, however, be anovulatory follicles. They may have a negative influence on the emergence of other follicular waves, but this influence seems to last for only approximately 8 days.9,21 As with other species, poor body condition, depressed energy intake, and decreased vitamin and mineral intake suppress reproductive activity in ewes and does. Lowered energy balance results in poor or weak signs of estrus, depressed ovulation, abnormal cycle, and delayed puberty. Deficiencies in energy, protein, vitamins A and E, phosphorus, and many trace minerals (iodine, copper) are commonly seen. These deficiencies are most commonly associated with irregular estrous cycles. The consumption of fescue infected with Neotyphodium coenophialum is associated with decreased reproductive efficiency. The ergot alkaloids have been shown to affect prolactin production in ewes and to increase the interval from introduction of the ram to conception. Sheep appear to be sensitive to the effects of phytoestrogens. Clinical observations include infertility, irregular and prolonged heat cycles, lowered conception rates, and early embryonic death. Fertility in lactating cows is decreased during the hot seasons of the year. Heat stress may cause decreased estrous detection, impair follicular development, disrupt function of the reproductive tract, affect oocyte competence, and lead to early embryonic death. Embryos develop resistance to heat shock as they age. Bovine morulae to blastocyst stages are unaffected by heat shock.22 Anestrus may be defined as ovarian inactivity. The causes of anestrus are multiple and include diseases of the reproductive and other systems. In addition, the problem is complicated by management factors that cause estrus to pass undetected, even though the animal’s estrous cycles and estrous behavior are normal. Common causes of anestrus in mares are summarized in Table 43-1. The mare likely undergoes puberty between the ages of 12 and 24 months. Because the horse is not an agricultural production animal, there has been little interest in studying the onset of puberty as in other species, where hastening puberty increases production. Most mares are not bred until they are at least 3 years old, thus making prepubertal status an unlikely differential diagnosis for infertility. The mare is a seasonally polyestrous animal, showing anestrus during the shorter days of the year and cycling regularly during the longer days. Length of anestrus varies from one to several months, although some mares, particularly in the tropics, may cycle year round.23 In California, Australia, and South Africa 18% to 25% of mares cycle year round.1,24–26 Anestrous mares may be indifferent to teasing and do not show regular estrous behavior. Ovaries are small and firm on palpation, and the uterus is flaccid with a thin endometrium. The cervix has mild tone and may be indistinct. On speculum examination of the vagina, the vaginal mucosa is pale and dry, and the cervix usually appears closed but is occasionally open or may be easily opened. Mares that experience seasonal anestrus will go through a transition period in late winter and early spring, characterized by the development of waves of antral follicles that regress without ovulation because the ovulatory surge of luteinizing hormone (LH) is absent.26 Transitional mares exhibit signs of estrus, including clitoral “winking,” tail flagging, and urinating in the presence of the stallion. Eventually, increasing LH concentration coincides with a large follicle, resulting in ovulation. After the first ovulation of the season, the mare will continue to ovulate on successive estrous cycles. A transition period is also observed during the fall as the mare changes from a polyestrous condition to the winter anestrus. Differential diagnoses for seasonal anestrus are listed in Table 43-1. As day length increases, most mares ovulate and begin regular cyclicity without treatment. Methods to advance the onset of regular ovulatory periods are discussed in the following sections. The vernal transition can be moved but not shortened beyond its physiologic length of 6 to 8 weeks by exposure of mares to artificial light. A common artificial lighting regimen is to expose the mares to 16 hours of light and 8 hours of dark by extending the photoperiod in the evening starting in late November to initiate ovulation by February (in the northern hemisphere). Light should be added to the end of the day, or split between the beginning and end of the day, as opposed to adding light only at the beginning of the day.26 An alternative regimen is to expose mares to 1 hour of artificial light 9.5 to 10.5 hours after the onset of darkness.27 Use of one 200-watt incandescent bulb or two 40-watt fluorescent tubes at a height of 7 to 8 feet in a 12- by 12-ft box stall has been recommended.28 Paddock lighting has been described. Treatment with GnRH or a GnRH analogue for mares in anestrus or spring transition has been shown to induce ovulation.29–33 Twice-daily injections of a GnRH agonist induced ovulation in a majority of mares within 2 to 3 weeks.33 Mares that are in deep anestrus (January and February, northern hemisphere) can be expected to return to anestrus after treatment. Domperidone and sulpiride have been reported to stimulate follicular activity and advance the first ovulation of the year in seasonally anestrous mares.34,35 However, the efficacy of dopamine antagonists in advancing follicular growth and ovulation in anestrous mares has recently been questioned, and it has been suggested that adjustments in light and climatic conditions may influence the efficacy of the treatment.36,37 Exogenous progestins suppress the release of LH from the anterior pituitary and may be used for estrous regulation during the vernal transition. After treatment of mares for 10 to 14 days, withdrawal of progestin may result in LH release from the pituitary and estrus beginning in 4 to 5 days, with ovulation within 10 days after cessation of treatment. Mares should be in mid to late transition and have a follicle at least 25 mm in size to respond to treatment. Progestins will not induce estrus or ovulation in anestrous mares.38 The recommended dose of progesterone in oil is 150 to 300 mg daily by IM injection. The synthetic progestin, altrenogest, is administered orally (PO) at 0.044 mg/kg daily. Progestins may be used in combination with extended photoperiod and gonadotropins. Products that are ineffective or unavailable include repositol progesterone, melengestrol acetate, chlormadinone acetate, proligestone, medroxyprogesterone acetate, hydroxyprogesterone acetate, and norgestomet implants. Synchronization of the first ovulation (or any ovulation during the cyclic season) can be accomplished by the administration of a combination of progesterone in oil (150 mg/day IM) and estradiol-17β (10 mg/day IM) once daily for 10 days.39 Treatment is most effective if given after mares have been under lights for 45 to 60 days. PGF2α should be given on the last day of steroid treatment. Treated mares will ovulate within 8 to 10 days after the last treatment. At a dose of 2500 to 3000 IU, hCG may induce ovulation within 48 hours when administered to a mare with a follicle larger than 35 mm in diameter40 and may reduce time to first ovulation in transitional mares, particularly when used in combination with lights and/or progesterone treatment. Ovulation response here is less predictable than that induced with hCG during the breeding season. Crude pituitary extract has been used to induce ovulation in anestrous mares.41 Recent data suggest that treatment of seasonally anestrous mares with recombinant equine FSH (reFSH) is effective in stimulating development of follicles and advancing the first ovulation of the year.42 Treatment with FSH resulted in multiple ovulations but was not successful in inducing continued cyclicity. Ultrasound-guided transvaginal follicular aspiration of follicles less than 35 mm has been shown to hasten the onset of cyclicity in transitional mares.43 Follicular aspiration resulted in the formation of an active CL, and subsequent treatment with PGF2α resulted in estrous behavior and ovulation of a dominant follicle. Mares that experience embryonic loss in the presence of endometrial cups (days 35 to 150 of gestation) are said to be pseudopregnant (pseudopregnancy may also refer to that condition in which a conceptus was lost after maternal recognition of pregnancy and before the development of endometrial cups, resulting in prolonged luteal life). In spite of the loss of the fetus and placental tissue, endometrial cups remain in place and continue to secrete equine chorionic gonadotropin (eCG) for a similar period to that in a pregnant mare, to 100 to 150 days of gestation.44 The primary and secondary CLs occasionally regress after embryonic loss45 but usually remain during eCG secretion, maintaining high levels of peripheral progesterone. Persistent endometrial cups throughout pregnancy and during the following breeding season have been described.46 In untreated mares, cyclic activity is often reestablished after the cessation of eCG secretion. Repeated daily injections of PGF products have been reported to cause luteal regression in pseudopregnant mares,47 but only CLs older than 5 days respond to the treatment, which may prevent mares returning to estrus. Pregnancies have occurred in the face of high eCG,48 but fertility of treated mares is usually low. Behavioral estrus may not be detected in otherwise normal mares as a result of inadequate estrous detection or a failure on the part of the mare to show obvious signs of estrus. The latter may occur in up to 15% of mares on well-managed farms.49 Inadequate estrous detection may be a result of human apathy or ignorance or a result of using a low-libido or inexperienced stallion. Teasing mares as a group may make detection of estrus more difficult, especially for nervous mares, mares with foals, and mares of low social rank. Use of anabolic steroids may suppress behavioral estrus. The mare fails to show estrus on adequate teasing with a stallion. Differential diagnoses are listed in Table 43-2. TABLE 43-2 Irregularities of the Equine Estrous Cycle: Differential Diagnosis AMH, Anti-müllerian hormone; CL, corpus luteum; PGF, prostaglandin F2α. Management should be examined to ensure a competent teasing routine. When approached by a stallion, mares in estrus stand still with ears held forward; they may elevate the tail, rhythmically evert the clitoris (“winking”), assume a squatting posture, urinate, and lean against the teasing chute toward the stallion. Mares that are in diestrus move about and hold their ears back; they may strike, kick, squeal, swish their tails, and forcefully void small amounts of urine. Experienced personnel should handle both stallion and mare. The teaser stallion should have adequate libido without being aggressive. Transrectal palpation and ultrasonography should supplement teasing. Some mares are indifferent to teasing, and records of sequential palpation must be relied on for breeding. Progesterone concentrations of less than 1 ng/mL are consistent with estrus but may also occur in anestrous mares. Mares that fail to show behavioral estrus should be bred by artificial insemination (AI) (if allowed by the breed register) or appropriate restraint used for natural cover. Abnormal estrous behavior and aggression may be demonstrated by otherwise normal mares at any stage of the estrous cycle. Cause is unknown, although exaggerated response to ovarian steroids has been proposed.50 Exaggerated signs of estrus occur, initially during estrus and then throughout the cycle. Mares may develop behavioral anomalies and become aggressive. Differential diagnoses are listed in Table 43-2. It is important to differentiate abnormal estrous behavior from unrelated behavioral problems. Exogenous progestins have been used to limited effect. Short-term dexamethasone treatment (5 to 10 mg) may alleviate signs for 3 to 4 days.50 Bilateral ovariectomy may be successful in some cases. Failure of a cow to display, or a manager to observe, the signs of estrus contributes significantly to reproductive inefficiency. When the presenting history suggests anestrus (failure to have a normal estrous cycle), the clinician must determine if the cause is failure of the manager to detect estrus in normal cows or failure of the cow to cycle because of some abnormal process. In dairy herds approximately 90% of cows presented for examination because of a history of anestrus have evidence of normal cyclic ovarian changes, whereas only approximately 10% are affected by an abnormality that suspends the estrous cycle (i.e., only ≈10% are in true anestrus). Nearly 90% of well-managed dairy cows have initiated normal-length estrous cycles by 60 days after calving, but only approximately 60% are detected correctly to be in estrus by that time.51 Rates of estrous detection by twice-daily observation range from 50% to 73% depending on the skill of the observer. Mounting activity and estrous behavior are reduced by hot and cold ambient temperatures and during the times of milking and feeding. More mounts are observed when cows are kept on dirt than on concrete. Estrous behavior varies with the time of day and may be an inverse reflection of extraneous activity interfering with cow behavior. In one study, 43% of cows showed heat between midnight and 6 am; 22% between 6 am and noon; 10% between noon and 6 pm; and 25% between 6 pm and midnight.52 Estrus in dairy cows averages 7 to 16 hours in length but ranges from 0.5 to 36 hours. Sixty-five percent of cows are in estrus for less than 16 hours, and 25% are in estrus for less than 8 hours.53 The number of mounts per hour ranges from 2 to 8, and the total number of mounts during estrus ranges from 11 to 56. Total number of mounts per estrus increases with the number of cows simultaneously in estrus. Dairy herds in which infertility is caused by inaccurate estrous detection are usually characterized by prolonged intervals from calving to first breeding and between services; insemination intervals of 10 to 15 days and 30 to 35 days; records of examinations that confirm cyclic ovarian changes, but in which observation of estrus is not recorded; and finding more than 15% of cows presented for pregnancy examination to be nonpregnant. Insemination during the luteal phase of the estrous cycle may occur in 10% to 20% of cows and is not likely to result in conception; insemination of pregnant cows may be followed by abortion. The diagnosis of unobserved estrus requires sequential examination of affected cows and accurate records. Other causes of anestrus are eliminated. Conditions such as CFD, pyometra, mummified fetuses, granulosa-theca cell tumors, and segmental aplasia that cause anestrus affect individual animals. Anestrus caused by undernutrition is characterized by depressed milk production and low body condition score. PGF is widely used in clinical management of unobserved estrus. Mature CLs (≈day 6 through day 18 of the estrous cycle) are responsive to PGF-induced luteolysis. Estrus occurs an average of 3 days (range 2 to 5 days) after administration of PGF, depending on the follicular status of the ovaries at the time of injection. The endocrine events surrounding the controlled estrus are indistinguishable from those surrounding spontaneous estrus and ovulation. Treatment with PGF shortens the intervals from treatment to first breeding and from treatment to conception but has no effect on fertility. The benefits of PGF treatment are limited by inaccurate palpation of the temporary ovarian structures, injection during the wrong phase of the cycle, and failure of the manager to observe estrus in treated cows (timed AI can be used to overcome this problem). Measurement of progesterone concentrations in milk samples taken on the day of breeding is useful in herds with a history of reduced fertility to confirm that cows being inseminated are not in the luteal phase of the estrous cycle. If more than an occasional cow presented for insemination has an elevated concentration of progesterone, the methods of estrous detection should be reviewed. Enzyme immunoassay kits for measuring concentrations of progesterone in milk and plasma of cows and other female animals have been described and are commercially available. Various heat detection aids have been developed. Several use devices mounted on the tailhead to record that a cow has stood to be ridden. Pressure-sensitive devices that are glued to the tailhead and change color after sustained pressure by the weight of a mounting cow are commonly used. Similarly, pressure-sensitive devices glued to the tailhead can send a record of riding events directly to a computer. Chalk, cattle crayon marker, or paint applied to the tailhead are inexpensive aids that are rubbed off when the animal is mounted when she is in heat. These methods require daily maintenance and twice-daily evaluation to function effectively. Detection aids that measure changes in activity (pedometers), mucous conductivity, or body temperature can be used successfully. Accuracy is enhanced when measurements are related to previous estrous activity and progesterone concentrations.54 Because unobserved estrus is primarily a problem of management, efforts to reduce time lost from delayed breeding are directed at improving efficiency of heat detection. Accurate records are required to identify cows that have not been observed in estrus by 40 days after calving. Cows not observed in estrus by 40 days after calving should be examined, and abnormalities of the reproductive organs that cause anestrus treated as indicated. The time of estrus can be predicted by palpation of the temporary ovarian structures, or estrus can be controlled with PGF. The most significant benefit of a planned herd health program is stimulation of improvements in management that decrease the interval from calving to conception as a result of improved estrous detection. Anestrus after insemination is frequently interpreted as a clinical sign of pregnancy. However, unobserved estrus in cows that have failed to conceive or have experienced early embryonic death (postservice anestrus) contributes significantly to increased calving intervals. Clinical management of postservice anestrus depends on diligent observation of cows 18 to 24 days after breeding and identification of nonpregnant cows as early as possible after the infertile service so that they may be reinseminated with minimum delay. Nonpregnant cows may be accurately identified by ovarian palpation or ultrasonography for absence of a mature CL, by low milk or plasma progesterone at the time of the first expected postservice estrus (≈21 days after breeding), or by palpation of the uterus per rectum before the second expected postservice estrus (30 to 42 days after breeding). Recently blood-based methods for pregnancy detection, including Pregnancy-Specific Protein B, have been validated as early as 28 days postinsemination with a sensitivity and specificity of 93.9% and 95.5%, respectively.12 The breeding season of most breeds of sheep maintained at temperate latitudes is restricted to late summer, autumn, and early winter, although some breeds cycle all year long. There is almost no homosexual interaction among ewes; therefore a male must be present to stimulate display of estrus.5 Introduction of a ram (either intact or vasectomized) into a flock of ewes advances the breeding season. Most ewes ovulate by 3 to 6 days after introduction of rams. The induced ovulation is seldom accompanied by estrus, but the subsequent estrus approximately 17 days later is ovulatory and fertile. The “ram effect” is lost when rams are allowed to associate with ewes throughout the year. Return to estrus after mating may be detected in a flock of ewes by fitting the ram with a brisket device that marks serviced ewes. Return of an excessive number of ewes to service after breeding alerts the owner to the possibility of infertility. AI of ewes is rare in the United States but more popular in other countries. Detection of estrus for AI depends on use of teaser rams mingled with the ewes or led through the flock several times daily. The breeding season of does is similar to that of ewes (i.e., it surrounds the autumnal equinox). During periods of short daylight, the normal estrous cycle of does is 20 to 21 days. Homosexual interaction among estrous does rarely occurs; so signs of estrus must be elicited by teasing. Signs of estrus may also be evoked by exposure to male pheromones by way of a “buck jar” prepared by rubbing a cloth over the scent glands caudomedial to the horns of a mature buck during the breeding season and storing the cloth in a tightly closed container. If estrus is not observed in does exposed to a mature buck or to a buck jar during the physiologic breeding season, pregnancy or pseudopregnancy might be considered as possible causes of anestrus. Severely parasitized or inadequately nourished does do not have normal estrous cycles. Deficiencies of phosphorus, iodine, and manganese have been suggested as causes of anestrus in does. Introduction of bucks into a flock of does early in the breeding season results in initiation of estrous cycles and some degree of synchrony of estrus approximately 10 days after introduction of the bucks.5 In contrast to ewes, however, the first ovulation after exposure to males is accompanied by estrus and fertile mating. Most female South American camelids, although showing signs of ovarian cyclicity as early as 5 months of age, have decreased fertility until approximately 15 months of age. A female camelid should be at least 60% of her expected adult weight before she is bred. Male camelids have a preputial attachment of the penis that is not separated until 2 to 3 years of age. Some males may actually detach as early as 15 months of age. Before this time they will show mounting behavior but will not be capable of intromission.9 Sexual differentiation occurs in three stages, each stage dependent on the previous one: Chromosomal sex is determined at fertilization. All normal mammalian oocytes contribute an X chromosome in addition to one of each representative autosomal maternal chromosome. Sperm cells contribute either an X or a Y sex chromosome in addition to one of each representative autosomal paternal chromosome. Abnormalities in chromosomal sex occur because of nondisjunctional errors during either mitosis or meiosis. Fig. 43-1 shows some examples of chromosomal sex anomalies. Monosomy X is also known as Turner syndrome. Owing to the lack of a Y chromosome and the consequent Sry gene, but the presence of the Dax1 gene on the present X chromosome, the phenotype is female. Monosomy X is the most commonly reported chromosomal abnormality in mares.1 Animals with this syndrome often have a history of poor performance and lack of or sporadic reproductive cyclicity. Ovaries are typically inactive, small, smooth, and firm. The uterus and cervix are usually hypoplastic. Externally the mare’s genitalia may appear normal or underdeveloped. The genotype for XXY syndrome is part of Klinefelter syndrome. Because of the presence of a Y chromosome and the consequent Sry gene, affected individuals are phenotypically male, but probably owing to the presence of two copies of the Dax1 gene on the two X chromosomes, they generally have hypoplastic genitalia and reproductive organs. It is thought that some factor (Dax1 or some other) on the X chromosome must escape the inactivation process, which happens early in development (around day 7 or 8 in the horse). Testicular development and spermatogenesis are inhibited, resulting in small, flaccid testes and azoospermia. The testes may be retained or descended, but they are often small and soft. The penis may be normal or smaller than usual. Affected males often show normal libido and sexual behavior. Low testosterone concentrations may be noted. Infertility always accompanies this syndrome. Reports have been made in numerous species, including the horse.55,56 More is not better. A report of an infertile mare with the 65,XXX genotype confirms this.57 The mare had bilaterally small, inactive ovaries and a hypoplastic uterus and cervix. Mosaics are individuals that have at least two cell lines with different karyotypes arising from the same zygote (Fig. 43-2). Phenotypes vary in accordance with the degree of mosaicism. Varying degrees of hermaphroditism and pseudohermaphroditism have been reported in many domestic species. Mosaics often have mixed gonadal dysgenesis, with an ovary and a testis, or ovotestes, owing to sex chromosome mosaic cell lines.58,59 These are true hermaphrodites. Chimeras are individuals having cell lines from two different embryonic sources. This can occur experimentally or from the natural fusion of blastocysts in utero. The possibility has been reported from a suspected double ovulation and fertilization followed by blastomere fusion in the horse (64,XX/64,XY and 63,XO/64,XY genotypes reported). Freemartinism is a common occurrence in ruminants, resulting in chimeric twins. Freemartinism is a phenomenon in ruminants in which an infertile female is twin to a male. The dizygotic occurrence happens when the blastodermic vesicles of the two zygotes fuse early in development (day 18 to 20 in cattle) and share embryonic tissue. The placentas fuse (day 30 to 50 in bovines), and they share blood throughout gestation. This occurs before gonadal differentiation at day 40 to 50. Both individuals are XX/XY chimeras. The Sry gene of the male twin causes the freemartin gonads to develop at least partially toward the male testis. The degree of differentiation varies with each freemartin, and many freemartin gonads remain undifferentiated. The shared circulation allows testosterone and anti-müllerian hormone (AMH; discussed later) from the male twin, and possibly from the chimeric freemartin, to affect the freemartin genitalia, and so she lacks a cervix, uterus, uterine tubules, and cranial vagina. The vulva is fairly normal. The yearling freemartin fails to exhibit estrus, the udder and teats remain small, and the freemartin externally resembles a steer (only with a vulva). Diagnosis can be made by establishing a blind end to the vagina (no cranial vagina, no cervix). Of heifers born co-twin to a male, 92% will be freemartins.60 The male twin may develop into a fertile adult, but these individuals show a higher incidence of infertility than bulls with a 60,XY genotype. Most male twins to freemartins become steers. Freemartinism is less common in sheep than in cattle, but it does occur. It has also been reported in goats and pigs. With increased fecundity in modern animals, the authors observe the phenomenon more often than was reported in the past. This is because ovine freemartinism is rare with twins or triplets but much more common with quadruplets or quintuplets. A notable difference between ovine and bovine freemartinism is the marked masculinity of the ovine freemartin. Gonads within the inguinal canal resemble normal prepubertal testes, and those within the abdomen resemble cryptorchid testes from rams of normal XY gonadal sex. Many ovine freemartins also have epididymides, vasa deferentia, vesicular glands, and even cremaster muscles. Chromosomal sex determines gonadal sex. The Y chromosome has very few genes, and all of the ones studied play a role in sex differentiation. The gene located at the sex-determining region on the Y chromosome (Sry) has a DNA binding domain high-mobility group (HMG) box. It produces a protein called the HY antigen, and its action appears to be regulated by the transcription of other genes. Their actions initiate differentiation of bipotential embryonic gonadal tissue into testicular tissue. Other genes act downstream of Sry to support gonadal differentiation, including Sox9, Gata4, and Wt1, and Sf1, which act synergistically to promote testicular differentiation. Sox9 is a powerful promoter of testicular tissue differentiation. It is hypothesized that Sry upregulates Sox9. In the absence of the Sry gene, the dual copies of the Dax1 gene on the X chromosomes suppress the formation of testicular tissue by antagonizing the Sry gene and the synergy between Sf1 and Wt1. Another gene, Wnt4, has been shown to support female development, and the absence of this gene in female mice results in masculinization.61 This evidence refutes the notion that female development is strictly a default process caused by the absence of Sry. Further research is elucidating other factors that actively promote the formation of ovarian tissue (e.g., Rspo1 β-catenin signaling). Factors affecting Sry, or any of the other genes downstream of Sry, or any factors governing female development will affect phenotypic sex. Sex reversals occur when the chromosomal sex and gonadal sex do not agree with each other. XX sex-reversed males and XY sex-reversed females are reported in many domestic species. These individuals are either XX males (testicular tissue), XY females (ovarian tissue), or true hermaphrodites (both ovarian and testicular tissue on separate gonads or the same gonad [ovotestes]). Sex reversals are relatively common in the horse and are considered to occur both sporadically and with familial inheritance. XY sex-reversed females have been reported to arise because of an absent or mutated (and nonfunctional) Sry gene. It is believed that the Sry chromosome is missing because of an abnormal meiotic exchange with the X chromosome. It is thought that in the sire two crossing-over events occurred between X and Y chromosomes during spermatogenesis. These animals are infertile and have a normal female appearance to their external genitalia. The ovaries and uterus tend to be hypoplastic. The gonads may, in fact, be completely undifferentiated (“streak gonads”). These animals may be true hermaphrodites (ovotestes) or XY females (only ovarian tissue). XX sex-reversed males conversely may arise from a translocation of the Sry gene onto the X chromosome. These animals may be true hermaphrodites or XX males (only testicular tissue). This condition has been reported in ruminants, but not horses. There are reports in horses of Sry-negative XX sex-reversed males.62,63 The exact mechanism of masculinization is still uncertain. Possibilities include Y-specific sequences other than Sry (which would require an XY individual with an inactivated or absent Sry gene), XX/XY chimerism within the testicular tissue, and a mutation in an autosomal or X-linked gene farther down the cascade of genes responsible for sex determination. XX sex-reversed males may have ambiguous sexual characteristics and may be true hermaphrodites or XX males (only testicular tissue). Goats (especially Alpine, Saanen, and Toggenburg) present another classic, common example of Sry-negative XX sex-reversed males. The “polled” (hornless) gene is either closely linked to an intersex locus or the polled gene itself is pleomorphic and controls both the hornless and intersex traits. This close linkage or pleomorphism is called the polled/intersex syndrome (PIS). A partial reason for the sex reversal is the deletion that affects a noncoding RNA (Pisrt1) and a transcription factor (FoxL2). The mechanism of testis induction has not been discovered yet. Elucidating this mechanism may be a big step in describing autosomal sex-determining factors in other species, including humans. The intersex gene is currently thought to work by mimicking the Sry gene and codes for the HY antigen. The polled gene shows a dominant autosomal inheritance pattern, whereas the intersex trait shows a recessive autosomal inheritance pattern. A single dose of the P polled gene is enough to cause the polled trait, but a double dose is required to cause intersexuality in XX individuals. Therefore PP animals are hornless and infertile (if XX, fertile if XY); Pp individuals are hornless and fertile; and pp individuals are horned and fertile. Intersex individuals are always sterile and have a shortened vagina, large clitoris, bucklike head and neck, buck odor, and buck behavior. The gonads are testes or ovotestes and may be scrotal, inguinal, or abdominal. Even very masculinized intersexes with scrotal testes are azoospermic. A PP polled goat that has an XY genotype is usually initially fertile but often develops sperm granulomas later in life. The only way to avoid the intersex condition is to always breed a polled individual to a horned individual (Fig. 43-3). Gonadal sex determines phenotypic sex. Initially each embryo has both müllerian (paramesonephric) ducts and wolffian (mesonephric) ducts. Within testicular tissue, Sox9 triggers Sertoli cells to secrete müllerian inhibiting substance (MIS; also known as anti-müllerian hormone [AMH]), which initiates the irreversible regression of the paramesonephric ducts. The action of MIS or AMH is further regulated by other genes and their proteins (Sf-1, Gata factors, Wt-1, Dax-1, and FSH). Wt-1 and Sf-1 synergistically enhance AMH transcriptional activity. Gata-4 enhances AMH promoter activity by directly binding to DNA and by synergistically interacting with Sf-1. Leydig cells secrete testosterone that is converted by 5α-reductase to dihydrotestosterone. These two steroids promote the differentiation of male genitalia. Testosterone influences the differentiation of the wolffian ducts into the internal male genitalia (vasa deferentia and epididymides), whereas dihydrotestosterone stimulates the formation of the seminal vesicles and male urethra from the urogenital sinus, and the penis from the genital tubercle. In the absence of these testicular hormones, the wolffian ducts regress and the müllerian ducts become the female internal genitalia. In the presence of two X chromosomes, the double dose of Dax-1 has an inhibitory effect on the synergistic relationships between Sf-1 and Wt-1 and Gata-4, preventing their support of AMH production.64 The primitive sex cords (gonadal cords) degenerate in the medulla and remain in the cortex (opposite in the horse). Subsequently there is no communication between the gonad and the mesonephros. In the absence of AMH, the müllerian ducts persist as the oviducts and fuse to form the uterus and cranial vagina. In the absence of testosterone, the wolffian ducts regress. Vestigial traces of these are located in the mesentery of the ovary, the epoophoron, the paroophoron, and Gartner ducts. The tissues that form the round ligament of the uterus are analogous to the male gubernaculum. Abnormalities of phenotypic sex occur when the chromosomal and gonadal sex agree (XX with ovaries or XY with testes) but the external and/or internal genitalia do not correlate or are ambiguous. Affected animals are the male and female pseudohermaphrodites. The condition can occur because of insensitivity of androgen receptors or because of any abnormality along the pathway that may affect the intervening hormones, such as the conversion of 5α-reductase to dihydrotestosterone. Testicular feminization is reported in domestic species, including the horse.65 Patients have external genitalia that are either female or ambiguous in appearance. The vagina may be blind-ending, or the uterus may be hypoplastic. The gonads are testicles, although they are usually abdominal or inguinal. Male behavior may be reported in horses. The problem lies in the gene for the androgen receptor, located on the X chromosome received from the dam. This X-linked recessive inheritance has been demonstrated in both humans and horses. Affected individuals are male pseudohermaphrodites; the condition has been diagnosed in multiple horse breeds. Serum testosterone is often elevated as a baseline because of loss of negative feedback, or at least in response to an hCG test. ▪ MARES The most common causes of bilaterally small ovaries in the mare are (1) seasonal anestrus, (2) hypothalamopituitary dysfunction, (3) severe malnutrition, (4) immaturity, (5) advanced age, (6) use of anabolic steroids, and (7) gonadal dysgenesis. Clinical signs and treatment of seasonal anestrus, hypothalamopituitary dysfunction, and malnutrition are discussed elsewhere in this chapter. The average age at which fillies reach puberty is 18 months, with a wide range of 10 to 24 months. Fillies younger than 2 years of age with inactive ovaries and a flaccid and relaxed reproductive tract may be too young to cycle and should be reexamined at a later time. The condition should be differentiated from gonadal dysgenesis, and karyotyping may be indicated if puberty is delayed beyond 24 months. Older mares (>20 years) not only frequently begin cycling later in the season than younger mares66 but also often have a longer follicular phase and, subsequently, a longer interovulatory interval.66,67 Additionally, older mares may have elevated serum gonadotropins and fewer ovarian follicles. Some mares reach ovarian senescence when they grow older66,68,69 and typically have small, inactive ovaries (follicles <5 mm) and a flaccid uterus and cervix. Although senescent mares may still show behavioral signs of estrus (similar to anestrous or ovariectomized mares), ovarian function is completely absent. Oocytes collected from aged donor mares, cultured, and transferred to young recipient mares were less likely to result in pregnancy than oocytes from young donor mares.70 Furthermore, oocytes obtained from old mares and examined using transmission electron microscopy had more morphologic abnormalities than oocytes from young mares. Results from these studies suggest that oocyte quality declines as mares age, and this factor will contribute to poor fertility in older mares. No treatment is available for age-related ovarian inactivity. Because aged mares often experience a delayed seasonal onset of ovarian activity, they benefit from an artificial light regimen starting 60 to 90 days before the breeding season. GnRH has been used to stimulate follicular growth in mares with poor follicular development attributable to anestrus or transition. Several studies (reviewed by Ginther26) have examined the effects of GnRH administered at different doses and intervals to stimulate follicular development in mares. In general, the number of mares responding to GnRH therapy increased as the size of follicle or time of year at the onset of therapy increased. Anabolic steroids are derivatives from androgens that have been altered to provide high anabolic activity with minimal androgenic side effects. A suppression of gonadotropin secretion has been documented when mares have been treated with these drugs.71 Anabolic steroid administration may affect both estrous behavior and ovarian size and function. The treatment of mares with low doses of anabolic steroids can cause aggressive or stallion-like behavior, whereas high doses can inhibit ovarian activity and result in failure of follicular development and ovulation.71 Prolonged treatment of prepubertal mares with anabolic steroids results in hypertrophy of the clitoris.72 Ovarian inactivity in mares treated with anabolic steroids is reversible, and pituitary and ovarian function eventually return to normal in most mares after withdrawal of the treatment. Mares intended for breeding should not be treated with anabolic steroids. Progestins are commonly given to cycling mares for the suppression of estrus or synchronization of ovulation. Mares may continue to ovulate during progestin administration, especially if treatment is started late in the luteal phase. A high incidence of persistent CL formation has been observed in mares that ovulate during progestin treatment.73 Administration of the potent GnRH agonist deslorelin acetate (Ovuplant, Fort Dodge Animal Health, Overland Park, Kan.) to induce ovulation has been associated with delayed follicular development and a prolonged interovulatory interval.74,75 Deslorelin acetate is effective in inducing ovulation, but treatment appears to cause a temporary downregulation of follicle stimulating hormone (FSH) secretion. The low FSH concentrations have been associated with a prolonged period of decreased follicular growth. Administration of PGF2α 7 to 8 days after ovulation appears to increase the risk of delayed follicular development. It has been suggested that PGF2α administration “resets” the timing of the estrous cycle during a period when limited follicular activity is present. Removal of the deslorelin implant after ovulation has been detected and will decrease the incidence of prolonged interovulatory intervals.76 Chromosomal abnormalities occur in all breeds of horses. Mares are usually small and phenotypically female. The ovaries are small, firm, smooth, and inactive. The tubular tract is thin and flaccid. Endometrial hypoplasia is a common finding. Diagnosis is confirmed by physical findings and karyotype. Mares with gonadal dysgenesis are sterile, and there is no treatment.
Diseases of the Reproductive System

Nonpathogenic Infertility
Breeding Season
Mares
Clinical Signs
Ruminants
Cows.
Sheep.
Goats.
Camelids.
Cystic Follicular Degeneration
Cows
Clinical Signs and Diagnosis
Clinical Pathology
Treatment and Prognosis
Spontaneous Recovery.
Luteinizing Hormone.
Gonadotropin-Releasing Hormone.
Prostaglandin F2α.
Manual Rupture.
Ewes and Does
Camelids
Poor Nutrition
Plant Toxicity
Ergot Alkaloids
Estrogen-Producing Plants
Heat Stress
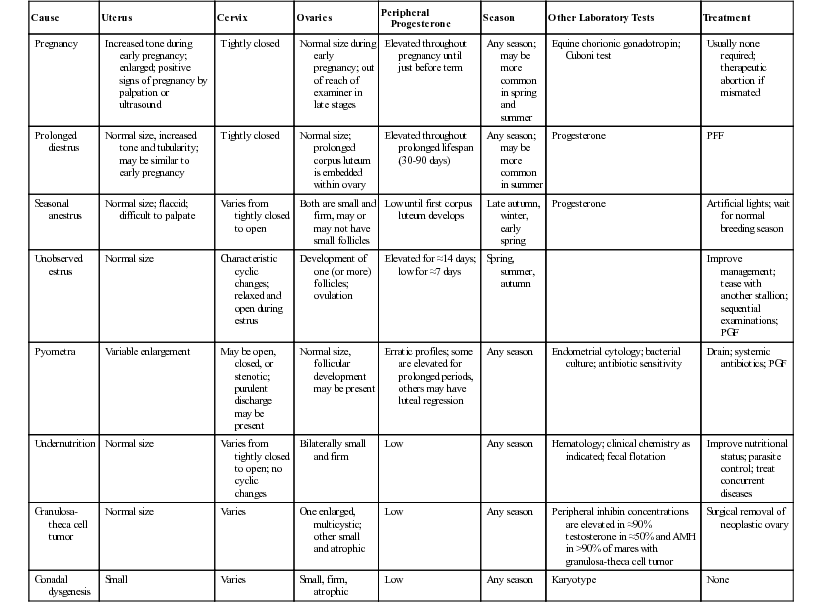
Anestrus
Mares
Puberty
Seasonal Anestrus
Clinical Signs and Diagnosis
Treatment and Prognosis
Artificial Lighting.
Gonadotropin-Releasing Hormone.
Dopamine Antagonists.
Steroids.
Gonadotropins.
Follicular Aspiration.
Prolonged Luteal Phase and Pseudopregnancy
Treatment and Prognosis
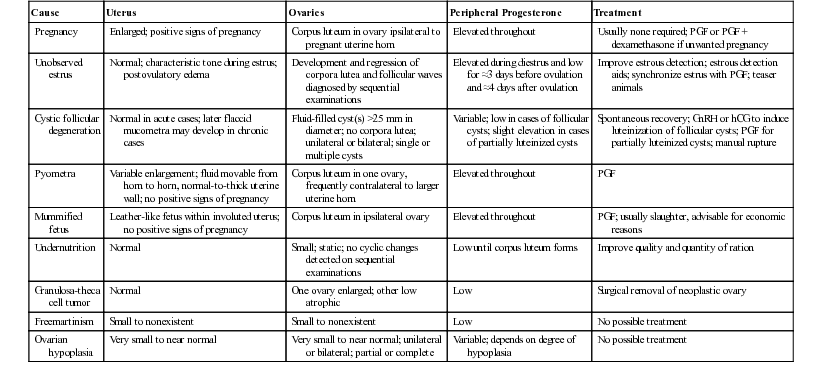
Lack of Behavioral Estrus (Silent Estrus)
Clinical Signs and Diagnosis
Etiology
Distinguishing Features
Failure to Cycle with Low Progesterone
Winter anestrus
Season; inactive ovaries
Gonadal dysgenesis
Small, hard, inactive ovaries; karyotype; underdeveloped tubular tract; small body
Pituitary adenoma
Systemic signs; inactive ovaries
Granulosa-theca cell tumor
See prolonged or irregular behavioral estrus
Behavioral
Intimidated by stallion; recently foaled; low social rank
Failure to Cycle with High Progesterone
Pregnancy
Presence of embryonic vesicle or fetus
Persistent CL
CL fails to regress; responds to PGF
Diestrous ovulation
CL immature at time of endogenous PGF; responds to exogenous PGF
Pseudopregnancy
Conceptus loss after maternal recognition of pregnancy; responds to exogenous PGF
Iatrogenic
History of exogenous progestin or nonsteroidal antiinflammatory drug administration
Pyometra
Uterus palpably enlarged
Short Luteal Phase
Uterine infection
Pyometra or endometritis causing premature endogenous PGF secretion
Systemic endotoxemia
Systemic signs; endotoxin-mediated release of endogenous PGF
Iatrogenic
History of uterine manipulation, infusion, invasive procedure, or exogenous PGF
Prolonged or Irregular Behavioral Estrus
Transitional period
Season, variable ovarian activity
Granulosa-theca cell tumor
Affected ovary large and multicystic, contralateral ovary small; elevated inhibin, AMH and/or testosterone; anestrus, nymphomaniac or stallion-like behavior
Gonadal dysgenesis
Occasionally irregular cyclicity; as above
Behavioral nymphomania
Otherwise normal mare
Normal mare
Mares in winter anestrus and pregnancy may show estrous signs
Treatment and Prognosis
Behavioral Nymphomania
Clinical Signs and Diagnosis
Treatment and Prognosis
Unobserved or Silent Estrus
Cow
Clinical Signs and Diagnosis
Treatment and Prognosis
Prevention and Control
Ewe
Doe
Camelids
Infertility Caused by Abnormalities of the Female Genital Organs
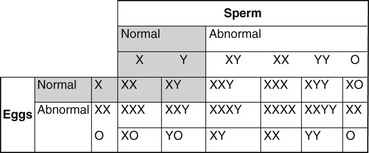
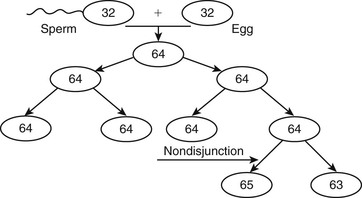
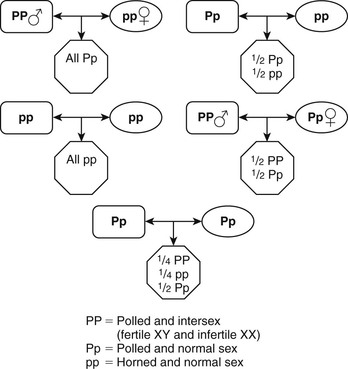
Abnormalities Caused by Problems with Sexual Differentiation
Monosomy X (XO)
XXY Syndrome
XXX
Mosaics.
Chimeras
Freemartins
Gonadal Sex
Abnormalities of Gonadal Sex: Sex Reversals
XY Sex-Reversed Females (Gonadal Dysgenesis).
Sry-Positive XX Sex-Reversed Males.
Sry-Negative XX Sex-Reversed Males.
Phenotypic Sex
Abnormalities of Phenotypic Sex
Testicular Feminization.
Abnormalities of the Ovaries
Abnormally Small Ovaries
Immaturity and Advanced Age
Treatment and Prognosis
Exogenous Hormone Treatment.
Treatment and Prognosis
Gonadal Dysgenesis.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Diseases of the Reproductive System
Chapter 43