Chapter 23 Disease Problems of Guinea Pigs
Gastrointestinal and Hepatic Diseases
Dental Disease
Dental disease is one of the most common veterinary presentations of guinea pigs. The anatomy of the guinea pig’s dentition, common clinical presentations of guinea pigs with dental disease, diagnosis, and dental treatment are discussed in detail in Chapter 32. Diets deficient in fiber or vitamin C, infection, and trauma are common reasons for malocclusion in guinea pigs; genetic predisposition while not proven, is also strongly suspected. A thorough oral examination should be part of the routine physical, as disease of the cheek teeth is easily overlooked. Because guinea pigs with dental disease often have concurrent disease processes, a thorough systemic evaluation is indicated before dental treatment is initiated. Perioperative supportive care is just as critical to a good outcome as the dental treatment itself; pain, hydration, nutrition (including vitamin C supplementation), and secondary infection must be thoroughly considered. Prevention is aimed at ensuring an appropriate high-fiber diet and vitamin C support.
Gastrointestinal Hypomotility or Stasis
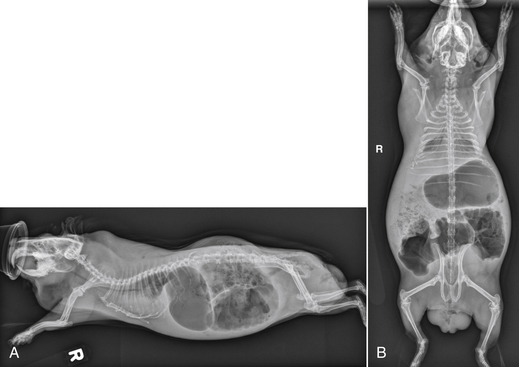
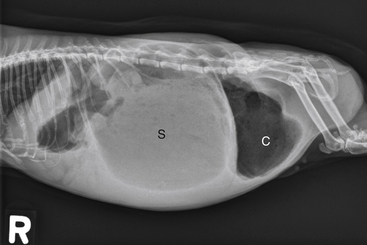
Clinical signs of GI stasis include decreased/absent fecal material, anorexia, bruxism, gas or fluid-distended stomach, cecum, and bowel loops, pain on abdominal palpation, decreased gastrointestinal sounds, and respiratory or cardiovascular compromise. The GI contents become dehydrated during stasis, exacerbating GI pain and anorexia and possibly leading to partial or complete GI obstruction. GI stasis can also result in the accumulation of gas within the intestinal tract (“bloat”), which can become life threatening (Fig. 23-1). Gastric dilatation-volvulus (GDV) has been reported in only two pet guinea pigs to date.11,33 A complete physical examination and appropriate diagnostics to evaluate for underlying disease are performed. Abdominal radiographs and ultrasound evaluate GI anatomy and motility. The clinical signs and physical exam findings of gastric/cecal dilatation/torsion are similar to those of GI stasis; imaging is required to distinguish the two conditions (Fig. 23-2). Blood work should be performed to evaluate for evidence of inflammation, infection, and organ dysfunction.
Regardless of the cause or severity, medical management of GI stasis consists of aggressive supportive care with replacement fluid therapy, pain management, and assisted nutrition. Hydrate the GI tract first to facilitate its motility and function during nutritional therapy. Currently several timothy hay-based critical care feeding formulas for small herbivores are commercially available. Supplement with vitamin C. Most guinea pigs tolerate syringe feeding very well. Some guinea pigs will not accept syringe feeding with a 60-mL catheter-tip syringe; patience is often required to feed small boluses of food with a 1- or 3-mL syringe. Nasogastric tube placement is difficult in guinea pigs; although finely ground critical care formulas that will pass through these small tubes are commercially available, it is difficult to provide the appropriate fiber particle size necessary to stimulate GI motility with these products.
Pain management is an essential component of therapy. GI pain is visceral and often too severe to respond to nonsteroidal anti-inflammatory drugs (NSAIDs) alone. The risks and benefits of using opioids are considered for each case, but guinea pigs with GI stasis usually respond well to buprenorphine or butorphanol. Prokinetics (metoclopramide, cisapride) should not be used initially in these cases, since partial or complete obstruction is common prior to hydration of GI contents. The use of commercially available probiotics or transfaunation has been advocated with prolonged stasis and when dysbiosis is suspected. Gastric decompression is performed on an emergency basis in cases of gastric tympany and is accomplished by passing a large-bore red rubber tube into the stomach through the oral cavity, being careful to not obstruct the glottis; however, gas lower in the GI system will not be affected. Gastric decompression can carry a high risk in the already debilitated patient, and often the volume of gas yielded is disappointing. Trocarization carries significant risk of gastric or cecal rupture or peritonitis and should be used only as a last resort. Surface tension relaxants such as simethicone anecdotally decrease the volume of accumulated gastric gas. Antibiotics should not be administered empirically in guinea pigs with GI stasis because of the guinea pig’s GI tract is highly sensitive to some antibiotics. Bacterial culture and sensitivity results or suspicions that the GI disease is caused by specific bacteria should be carefully weighed in deciding on antibiotic use. Surgery is rarely required for GI hypomotility/stasis, but if GDV is highly suspected, this is a surgical emergency. No information is available regarding prognosis and surgical management in guinea pigs with GDV, as all published reports have been based on diagnosis at necropsy.11,33
Dysbiosis and Antibiotic-Associated Enterotoxemia
Guinea pigs possess a predominantly gram-positive GI flora and are exquisitely sensitive to some antibiotics, which eradicate that flora. Penicillin, ampicillin, chlortetracycline, clindamycin, erythromycin, and lincomycin can destroy the most susceptible gram-positive organisms, permitting overgrowth of gram-negative bacteria and Clostridium difficile, with elaboration of its toxin.10,30 Toxin production causes hyperactivity of secretomotor neurons, resulting in secretory diarrhea and hemorrhagic typhlitis.55 This occurs most commonly when these antibiotics are given orally; however, it can also occur in some animals when given parenterally as well as with appropriate but prolonged antibiotic therapy. Non-antibiotic-induced dysbiosis also occurs and is generally associated with abrupt dietary changes, ingestion of contaminated foods, and stress. Anorexia, dehydration, and hypothermia are the most common clinical signs; diarrhea may or may not be present.30 Diagnosis is usually based on history, clinical signs, and histopathologic lesions. C. difficile is difficult to isolate, but polymerase chain reaction (PCR) and enzyme immunoassay for its toxin are commercially available. Antibiotic-associated enterotoxemia is treated symptomatically. Provide thermal support and administer intravenous or subcutaneous crystalloid fluids to restore hydration. Commercially available probiotics (Lactobacillus species) live-culture yogurt, or cecotrophs transfaunation from other guinea pigs can be used to try to reestablish normal microflora. Chloramphenicol (50 mg/kg PO q8h) may be somewhat effective in suppressing further clostridial overgrowth.10 Antibiotic-associated enterotoxemia is prevented by treating diseases in guinea pigs with appropriate antibiotics. Trimethoprim-sulfa, chloramphenicol, and enrofloxacin are generally safe in guinea pigs.
Enteritis and Diarrhea
Bacterial enteritis is also a common cause of soft stools/diarrhea. Tyzzer’s disease, caused by Clostridium piliforme, is transmitted by the fecal-oral route. Young, stressed, or immunocompromised animals are particularly affected. Clinical signs include lethargy/anorexia, diarrhea, unthrifty appearance, and acute death.30 Lesions observed at necropsy include intestinal inflammation and focal hepatic necrosis. C. piliforme is an intracellular bacterium and does not grow on routine culture media. Definitive diagnosis is by histopathologic identification of organisms from liver or intestinal sections. Treatment is generally unrewarding. The disease can be prevented by good husbandry practices and stress reduction, particularly at weaning.
Salmonella typhimurium and Salmonella enteritidis are less frequently reported causes of bacterial enteritis in pet guinea pigs, but mortality can be high during an outbreak. Transmission is usually due to fecal contamination of feed or water. Weanlings, pregnant sows, aged animals, and those with nutritional deficiencies are particularly susceptible. Signs include scruffy hair coat, weight loss, weakness, conjunctivitis, and abortion. Diarrhea may or may not be present. At necropsy, the spleen and liver are often enlarged, and yellow necrotic foci may be present in the viscera.41 Diagnosis is by fecal or intestinal culture and sensitivity testing. Treatment is usually not recommended, as affected animals can become asymptomatic carriers and salmonellosis can be zoonotic. Prevention is aimed at proper disinfection/sanitization of the environment, storing of food in airtight containers, and thorough washing of all fresh fruits and vegetables offered.
Other causes of bacterial diarrhea include Yersinia pseudotuberculosis, Clostridium perfringens, Escherichia coli, Pseudomonas aeruginosa, Citrobacter freundii, and Listeria monocytogenes.41 Like Salmonella species, these are usually contracted through food contamination. Y. pseudotuberculosis can cause abscesses of the intestine, liver, and regional lymph nodes.41 E. coli causes wasting, depression, and death in weanlings. Intestines may contain yellow fluid.
Guinea pigs are hosts to Eimeria caviae, Balantidium caviae, and Paraspidodera uncinata. In juvenile animals, E. caviae causes diarrhea, but this is much less common in adults. Cryptosporidium wrairi causes failure to gain weight, weight loss, diarrhea, and death, especially in weanlings and immunosuppressed animals.9 Transmission is via the fecal-oral route. Immunocompetent animals recover within 4 weeks and are resistant to reinfection. Organisms can be identified on fecal examination or by histopathology of the brush border of mucosal epithelial cells. No treatment has proved effective. Oocysts of C. wrairi can be destroyed in the environment with a 5% ammonia solution, freezing to below 32°F (0°C), or heating to above 149°F (65°C). Cryptosporidiosis is a potentially zoonotic disease.
Fecal Impaction
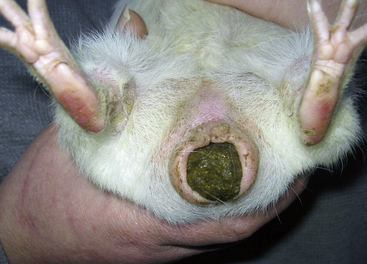
Fecal impaction is most commonly identified in older guinea pigs, especially boars, but the etiology is unknown (Fig. 23-3). Soiled bedding combined with inguinal sebaceous secretions can become adhered to the scrotum and anus, potentially resulting in inguinal gland infections and fecal impaction. The guinea pig is usually presented for straining to defecate, constipation, or passing large amounts of foul-smelling soft stool. Affected animals may appear to be uncomfortable when straining to push out the accumulated feces. Often the only physical exam abnormalities detected will be an enlarged rectum impacted with normal, soft feces and a flaccid anus. Suggested therapy has included a diet change to increase fiber, warm-water enemas, and manual evacuation of stools using a cotton-tipped applicator (often long-term therapy is required).
Bacterial and Viral Pneumonia
Bacterial pneumonia is one of the most important diseases of guinea pigs. Poor husbandry conditions mentioned above, along with humid environments, predispose to pneumonia.38 The most common etiologic agents are Bordetella bronchiseptica, Streptococcus pneumoniae, Streptobacillus moniliformis, and Haemophilus species.14,38 Regardless of etiology, the general progression of clinical signs is as follows: ocular/nasal discharge, dyspnea, increased respiratory sounds, sneezing, coughing, depression, anorexia, weight loss, and unthrifty appearance of the coat.14,38 Diagnosis includes clinical signs, physical exam findings, thoracic radiographs, and culture and sensitivity of respiratory secretions. Bacterial culture of the nasal and/or ocular discharge is often inconsistent with tracheal or bronchial cultures (causative organisms). Diagnostic samples are best retrieved from tracheal secretions, bronchoalveolar lavage, or fresh necropsy specimens from the bronchopulmonary region in colony outbreaks.21,38 Opacity within the tympanic bullae or lungs may be seen on radiographs in guinea pigs with respiratory disease.38 Thoracic auscultation may reveal crackles, rales, and wheezing or minimal air movement, depending on the severity of the disease. Systemic antibiotic therapy based on culture and sensitivity results and supportive care (fluid and nutritional support) should be instituted. Nebulized antibiotics can be considered for upper respiratory therapy. Supplemental oxygen, saline nebulization, bronchodilators, and decongestants should be considered as needed. Vitamin C supplementation is always a prudent addition to infectious disease treatment.
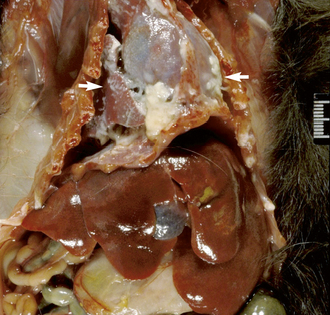
B. bronchiseptica is a gram-negative rod that is an important cause of respiratory disease. Rabbits, dogs, and nonhuman primates may be asymptomatic carriers. The disease is characterized by purulent bronchopneumonia often involving consolidated lung lobes and fibrinosuppurative pleuritis (Fig. 23-4). B. bronchiseptica infection may also cause exudates in the tympanic bullae, metritis, and abortions. Treatment is with antibiotics based on culture and sensitivity results. Prevention with an injectable B. bronchiseptica vaccine (Bronchicine, Bicor Animal Health, Omaha, NE; 0.2 mL SC with a booster 2-3 weeks later) has been used with moderate success in the face of an outbreak (C. Bishop, personal observation). It is recommended to prevent exposure of guinea pigs to rabbits and dogs that might be asymptomatic carriers of Bordetella.38
S. pneumoniae is a gram-positive coccus that may be transmitted by asymptomatic carriers of many species, including guinea pigs. Serotypes III, IV, and XIX cause disease in guinea pigs.18 Disease is predisposed by poor husbandry and is most common in young cavies. Bronchopneumonia, fibrinopurulent pleuritis, and pericarditis are common manifestations of this infection. One of the present authors (CB) has experienced an outbreak of S. pneumoniae in a caviary of 90 to 100 animals in which symptoms were not limited to the respiratory system. Metritis, encephalitis, and pericarditis were also identified in necropsy specimens. Animals affected by this outbreak had intermittent respiratory signs, infertility, abortion, death of young and sows after parturition, as well as neurologic signs including blindness, ataxia, and paralysis. Treatment with various antibiotics (based on culture and sensitivity results) led to resolution in only some individuals. Some animals initially improved from respiratory signs but succumbed to neurologic disease a few weeks later.
Outbreaks of adenoviral pneumonia have been reported in laboratory colonies,14 but the incidence in pet or exhibition animals is unknown. Adenoviral pneumonia causes a necrotizing bronchopneumonia with an incubation period of 5 to 10 days.38 Although morbidity is low, mortality is high and cavies may die acutely.
Urinary Diseases
Urolithiasis
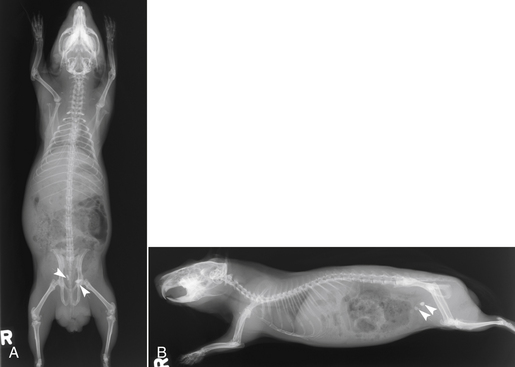
Urolithiasis is a common problem in guinea pigs. Historically middle-aged to older (>2.5 years old) female guinea pigs were overrepresented in the literature,13,40 but a recent study of 75 guinea pigs with calculi found an equal distribution of males and females >2 years.20 The etiopathogenesis is unclear, although the alkaline pH and high mineral content of normal guinea pig urine may favor crystal formation and precipitation. Of the few published reports of this disease in guinea pigs, the majority have been single case reports.15,48,50,52 Historical information suggested that calcium oxalate calculi were most common,37,48 but recent data have more commonly identified calcium carbonate calculi.13,15,20,50 Urinary tract infections involving E. coli, Streptococcus pyogenes, and Staphylococcus species were suggested to be associated with the presence of urinary calculi in laboratory guinea pigs.37,40 Pure cultures of Corynebacterium renale and Facklamia species and mixed bacterial cultures including C. renale, the Streptococcus bovis/equinus group, and Staphylococcus species were found from urine of pet guinea pigs with urolithiasis, although a direct association was not confirmed.13,20 Also, Streptococcus viridans, Proteus mirablis, and mixed growth with S. viridans, Staphylococcus species, E. coli, or Enterococcus species were reported from cultures from some of the calculi.20 The majority of calculi are located in the bladder or urethra, but they are also found in the kidneys, ureters, or vagina or sometimes in the seminal vesicles in males. Stones located in the ureter have been primarily identified from male guinea pigs.20 Affected guinea pigs were more likely to be fed a diet high in overall percent pellets, low in percent hay, and a lesser variety of vegetables and fruits.19 Alfalfa-based pellets and hay contain higher concentrations of calcium; it has been suggested this may also contribute to urinary calculi in guinea pigs.
Diagnosis is based upon clinical signs, physical examination findings, imaging studies including radiographs, ultrasonography, excretory intravenous pyelograms (IVPs), computed tomography (CT), and urinalysis. Urinary calculi in guinea pigs are generally radio-opaque, allowing for ease of identification using survey radiography (Fig. 23-5). However, if multiple calculi or significant GI gas is present, the anatomic locations of the calculi using survey radiography alone may be obscured. Ultrasound is useful for anatomic location of the calculi and for evaluating anatomic changes in the kidneys or ureters, such as hydronephrosis or hydroureter, ureteral mucosal inflammation, or perforation. Excretory IVPs are useful to further elucidate relative functional abnormalities in the kidneys or ureters. In some institutions, CT has virtually replaced excretory IVP, as images can be obtained more rapidly using significantly less contrast material. However, even though the time to image collection is longer, the excretory IVP can be performed at a reduced cost in any veterinary practice.
In suspected cases of urolithiasis, perform a urinalysis, including both a standard dipstick and microscopic examination of the sediment. Urinalyses data from 44 guinea pigs with urinary calculi found that the mean ± SD urine specific gravity was 1.015 ± 0.008 (range 1.004-1.046) and urine pH was 8.4 ± 0.5 (range 6.5- > 9). Hematuria was the most commonly reported abnormality on urine sediment, followed by mucus and lipid droplets.20 Calcium carbonate, calcium oxalate, and struvite crystals are all commonly seen on sediment examinations but, as in other small animals, crystal type(s) may not predict the mineralogy of the calculi.20 Prior to antibiotic use, culture the urine or bladder wall if high numbers of red or white blood cells, bacteria, or a combination of these are present on examination of the sediment. Collect urine via cystocentesis, as free-catch samples are commonly contaminated. Even gentle expression of the bladder can lead to hematuria. Calculi containing calcium carbonate require specific methodologies to differentiate calcium carbonate crystals from calcium oxalate monohydrate (COM) crystals; therefore the laboratory chosen for analysis must be able to perform these differentiating methods.20 Calcium carbonate calculi are not found in humans; thus human and some veterinary (those that do not commonly evaluate large/exotic animal samples) laboratories do not use techniques for differentiating calcium oxalate and calcium carbonate. Confirm the lab’s ability to perform these techniques in advance to ensure that the calculus is appropriately evaluated for the guinea pig patient.
Medical treatment of urolithiasis has been unrewarding to date, and surgical removal of the stones is most often required (see Chapter 25). In females, with the patient under deep sedation and analgesia, gently flush the bladder using a 3.5-Fr red rubber catheter to attempt to remove the calculi. Catheterizing boars is much more dangerous because of the small size of the urethra. Submit the calculi for analysis and culture and submit the bladder wall for culture and sensitivity. Postoperative management includes assisted feeding, fluids, analgesics, and antibiotics based on culture and sensitivity results. However, recurrence of the disease is common. Prevention is targeted at increasing water intake and reducing (but not eliminating) dietary calcium. We have seen metabolic bone disease (fibrous osteodystrophy; see “Musculoskeletal Diseases,” below) induced in guinea pigs with severe dietary calcium restriction for prevention of urinary calculi. For most animals, water is the cornerstone of any prevention protocol. Hydrochlorothiazide (2 mg/kg q12h) is a thiazide diuretic that reduces urinary Ca2+, K+, and citrate. It is unknown if diuresis would be of benefit to guinea pigs whose urine is already considered isosthenuric.20 Do not use hydrochlorothiazide with severe renal disease or fluid imbalances. Avoid alfalfa-based diets. Diets containing a high percentage of timothy, oat, or grass hays, a lower overall percentage of pellets, and a wide variety of vegetables and fruits decrease the risk of urolith development in pet guinea pigs.19 It is possible that dietary inhibitors of calcium are found in greater concentration in hays than in pellets. Urinary acidifiers were historically recommended based upon an assumed diagnosis of calcium oxalate calculi, but the normally alkaline urine of guinea pigs making the use of dietary acidifiers concerning. Potassium citrate (30-75 mg/kg PO q12h) binds urinary Ca2+, reduces ion activity, and alkalinizes the urine. Because guinea pigs have alkaline urine even with disease, the efficacy of this treatment is unclear. Hyperkalemia occurs, so monitor plasma K+ closely during treatment. According to anecdotal reports, potassium citrate and hydrochlorothiazide have been used together with some clinical success.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree