CHAPTER 23 Digestive System, Liver, and Abdominal Cavity
Approach to the Vomiting Cat
Vomiting begins with retching, a series of brief negative intrathoracic pressure pulses that coincide with positive abdominal contractions. These pressure changes occur as a result of repeated herniations of the abdominal esophagus and cardiac portion of the stomach into the esophagus. During retching, food freely moves back and forth in the esophagus, which is now dilated because of the ingesta. Ultimately, the diaphragm rapidly moves cranially, resulting in positive intrathoracic pressure that leads to expulsion of these contents.12 Vomiting is such an active process that it seems to involve the whole cat, and so it is little wonder that it concerns owners so much.
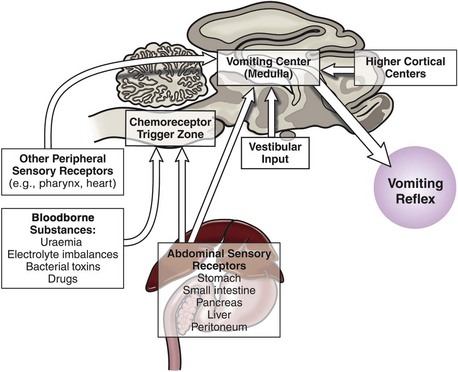
Since vomiting is mediated by the CNS with input and influence from just about anywhere in the body, it is important to summarize this physiology so it can be appreciated when managing clinical cases. Vomiting results from stimulation of the “vomiting center,” which is located in the brainstem; there are four main pathways that stimulate the vomiting center,12 and these are summarized below and in Figure 23-1.
1 Peripheral sensory receptors
2 Bloodborne substances can stimulate the chemoreceptor trigger zone (CTZ). The CTZ lacks a blood-brain barrier so that substances diffuse to it freely.
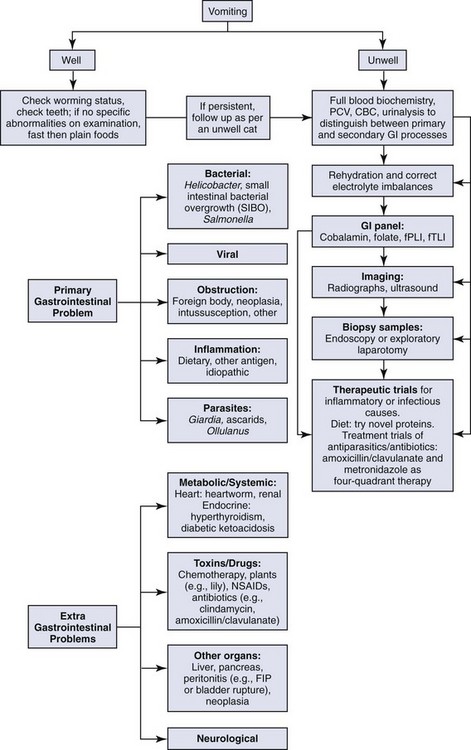
These complex pathways highlight the need to consider the whole cat and not just the cat’s gastrointestinal tract when assessing a cat presenting for vomiting. The approach to managing a cat with vomiting must follow logical steps. When the underlying cause is gastrointestinal disease, a precise diagnosis can only be reached after obtaining biopsy samples. A summary of diagnostic steps and possible underlying causes is shown in Figure 23-2.
Step 1: Signalment and Clinical History
The important aspects of the clinical history are given in Table 23-1. Signalment is important, because younger cats are more likely to have ingested foreign bodies (though not all older cats have grown out of this habit). Some extragastrointestinal problems, such as hyperthyroidism and renal disease are more likely to occur in older cats.
TABLE 23-1 Clinical History for Vomiting Cats
| Question | Interpretation |
|---|---|
| Signalment | |
| Diet | |
| Environment | |
| Duration and frequency | |
| Relationship to eating | |
| Describe vomiting process | |
| Appearance of vomitus | |
| Deworming history | |
| Previous illnesses | |
| Current medications | |
| Behavioral changes | |
| Co-existing systemic signs |
Adapted from Hall JE: Clinical approach to chronic vomiting. In August JR, editor: Consultations in feline internal medicine, ed 3, Philadelphia, 1997, Saunders, p 63.
Most texts and references instruct clinicians to distinguish between vomiting and regurgitation, with the latter noted as being quite passive.3,11,12 In practice, it can be hard to make this distinction, because it is the author’s experience that cats with esophageal disease can have quite forceful, spasmodic movements when ejecting ingesta by regurgitation—although it is also possible for regurgitation to be a passive process. Given that the physiology of vomiting, as described above, results in ingesta being forced to and then evacuated from the esophagus, it is hardly surprising that it can resemble regurgitation. Fortunately, regurgitation and esophageal disease do vary from vomiting in other ways! Vomiting is usually preceded by the cat licking its lips, salivating, or making attempts to swallow. Regurgitated ingesta is often in a tubelike structure and if undigested can be covered with frothy saliva. Partially digested food suggests vomitus, and the presence of bile or digested blood confirms this.
It is important to determine if the cat vomits regularly. Many owners have seen their cats vomit on a regular basis with no evidence of the cat being unwell, and this is noted frequently in the veterinary literature.3,12 Hairballs can cause gastric irritation, and it may be that eating quickly also stimulates the peripheral sensory receptors that contribute to vomiting. If a cat does vomit regularly, it is important to assess if the cat is presenting for a change in the vomiting pattern (e.g., frequency or timing in relation to eating) and if the cat is unwell in any way, such as anorexia or weight loss.
Step 2: Physical Examination
Vomiting is the major sign of gastric disease, but given the number of potential organ systems that can be involved, a thorough physical examination should be undertaken. Because linear foreign bodies are a common cause of vomiting, all cats presenting for anorexia or vomiting should have the underside of the tongue evaluated for the presence of string caught there. Applying gentle pressure with a thumb in the intermandibular space to elevate the tongue is an effective way to visualize lesions or foreign bodies in the sublingual area (see Figure 3-8).
Step 3: Blood and Urine Testing
Blood Tests for Gastrointestinal Disease
Cobalamin, folate, feline trypsin-like immunoreactivity (fTLI), and feline pancreatic lipase immunoreactivity (fPLI) tests are useful markers of intestinal and pancreatic disease,7,8,9,10 but it is important to note that they mostly do not give a precise diagnosis. More detail about the utility of these tests is noted below in the section Approach to the Cat with Diarrhea.
Step 4: Imaging: Ultrasonography and Radiology
Radiography is most useful for identifying foreign bodies or signs of intestinal obstruction from other causes. The major findings are noted below in the section Intestinal Obstruction. Contrast radiography can aid the diagnosis for both discrete and linear foreign bodies but should be used with caution, because intestinal perforation may be present. Nonionic iodinated agents that are typically used for myelography (such as iopamidol or iohexol) should be used, since barium irritates the peritoneum and oral iodine compounds are hypertonic. Hypertonic compounds may draw fluid into the stomach and intestines after oral administration, with the potential of creating further fluid and electrolyte imbalances in an already compromised patient.6
Step 5: Intestinal (and other Organ) Biopsies
Histologic evaluation of affected tissue is usually needed for diagnosis of most chronic gastrointestinal diseases. Intestinal biopsy samples can be obtained by the use of endoscopy, laparotomy, or laparoscopy, each of which has advantages and disadvantages. Laparotomy allows gross examination of and access to the entire intestinal tract as well as other abdominal organs. Laparotomy is the most invasive alternative, but with careful anesthesia and analgesia, many cats recover uneventfully. One survey assessed that 83% of cats undergoing exploratory laparotomy survived the hospitalization, and although complications occurred in 26% of cats, these were more likely to be associated with the underlying disease process and not surgery or anesthesia.4
Endoscopy is the least invasive procedure and is the only alternative that allows examination of the intestinal lumen. This option limits the parts of the gastrointestinal tract that can be biopsied; it does not allow examination or sampling of any other part of the gastrointestinal tract and does not enable full-thickness biopsy samples. One study found that, of cats investigated for gastrointestinal disease, 9 of 33 cats (27%) had no pathology recognized proximal to the jejunum (i.e., the effective length of diagnostic endoscopes would have precluded diagnosis), and other organs were affected in 9 of 10 cats with inflammatory bowel diseases and 7 of 8 cats with intestinal small cell lymphoma.1 Careful case selection for endoscopy from survey ultrasonography can reduce the number of missed diagnoses from endoscopy, but the possibility still remains.
The quality of endoscopically obtained biopsy samples varies greatly with the skill of the endoscopist. It has been stated that “it is exceedingly easy to take inadequate tissue samples with a flexible endoscope.”5 In an assessment of endoscopically obtained biopsy samples, two laboratories were compared, one that received samples from any practitioner and the other that received samples ONLY from practitioners trained to take, mount, and submit endoscopy samples. All slides were reviewed by three pathologists who found that, of samples from the first laboratory, 15% of the slides were considered inadequate for diagnosis, 71% were considered questionable, and only 14% were adequate. By comparison, in the second laboratory (with samples from experienced practitioners) 0% of slides were inadequate, 21% were questionable, and 79% were considered adequate for diagnosis.13 In the case of distinguishing between lymphocytic intestinal infiltrates (commonly known as inflammatory bowel disease) and lymphocytic neoplasia (small cell lymphoma), endoscopically obtained samples can give an incorrect diagnosis.2 Many of these problems can be minimized with experienced operators and careful case selection from prior ultrasonography.
1 Baral RM. Laparotomy for gastro-intestinal biopsies, Science Week Conference Proceedings (Small Animal Medicine chapter). Gold Coast, Queensland, Australia: Australian College of Veterinary Scientists; 2006. p 70
2 Evans SE, Bonczynski JJ, Broussard JD, et al. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447.
3 Hall J. Clinical Approach to chronic vomiting. In: August J, editor. Consultations in feline internal medicine. ed 3. Philadelphia: Saunders; 1997:61.
4 Lester S, Welsh E, Pratschke K. Complications of exploratory coeliotomy in 70 cats. J Small Anim Pract. 2004;45:351.
5 Mansell J, Willard MD. Biopsy of the gastrointestinal tract. Vet Clin North Am Small Anim Pract. 2003;33:1099.
6 Shaiken L. Radiographic appearance of linear foreign bodies in cats. Vet Med. 1999;94:417.
7 Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med. 2001;15:26.
8 Steiner JM, Williams DA. Serum feline trypsin-like immunoreactivity in cats with exocrine pancreatic insufficiency. J Vet Intern Med. 2000;14:627.
9 Steiner JM, Wilson BG, Williams DA. Development and analytical validation of a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2004;68:309.
10 Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203.
11 Tams TR. A diagnostic approach to vomiting in dogs and cats. Vet Med. 1992;87:785.
12 Twedt DC. Diseases of the stomach. In: Sherding RG, editor. The cat: clinical diseases and management. ed 2. New York: Churchill Livingstone; 1994:1181.
13 Willard MD, Lovering SL, Cohen ND, et al. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. J Am Vet Med Assoc. 2001;219:474.
Therapeutics for Vomiting and Diarrhea
Nonspecific Supportive Therapies for Vomiting
Antiemetics and Prokinetics
Antiemetics and prokinetics are used to control or prevent vomiting through specific receptor interactions mediated either centrally or peripherally, making some more effective in cats than others. The five most commonly used antiemetics all control vomiting by different mechanisms and include mirtazapine, metoclopramide, dolasetron/ondansetron, maropitant, and the phenothiazines (Tables 23-2 and 23-3). Metoclopramide functions both as an antiemetic and prokinetic in cats, while cisapride functions solely as a prokinetic.
TABLE 23-2 Mechanism of Action and Adverse Effects of the Common Antiemetic and Prokinetic Drugs Used to Treat Vomiting in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Metoclopramide (antiemetic and prokinetic) | D2 antagonism 5-HT3 antagonism 5-HT4 agonist | Extrapyramidal signs |
| Dolasetron (antiemetic) Ondansetron (antiemetic) | 5-HT3 antagonism | Prolongation QT interval Arrhythmias |
| Maropitant (antiemetic) | NK-1 antagonist | |
| Phenothiazines (antiemetic) Prochlorperazine Chlorpromazine | D2 antagonism H1, H2 antagonism Cholinergic antagonism alpha1, alpha2 antagonism | Extrapyramidal signs Sedation Decreases seizure threshold Hypotension |
| Cisapride (prokinetic) | 5-HT4 agonist | Prolongation QT interval Arrhythmias |
| Mirtazapine (appetite stimulant and antiemetic) | 5-HT2 , 5-HT3 antagonism H1 antagonism | Behavior changes Tremors, muscle twitching Hyperactivity |
TABLE 23-3 Dosage Recommendations, Contraindications, Potential Drug Interactions, and Clinical Indications for Dosage Adjustments for the Common Antiemetic and Prokinetic Drugs Used to Treat Vomiting in Cats
| Drug | Dosage (Cats) | C: Contraindications DI: Drug Interactions DA: Dosage Adjustments |
|---|---|---|
| Metoclopramide | 0.2-0.4 mg/kg SC, PO q8h 1-2 mg/kg/day CRI | C: GI obstruction DI: Phenothiazines: extrapyramidal signs DA: Azotemia |
| Dolasetron Ondansetron | 0.5-1.0 mg/kg IV, SC, PO q12-24h 0.22-0.5 mg/kg IV, PO q8-12h | DI: Cisapride: prolonged QT interval and arrhythmias |
| Maropitant | 1 mg/kg IV, SC, PO q24h (up to 5 days) | |
| Phenothiazines prochlorperazine chlorpromazine | 0.2-0.4 mg/kg SC q8h | C: Dehydration; hypotension; seizure hx DI: Metoclopramide: extrapyramidal signs |
| Cisapride | 1.5 mg/kg PO q12h | C: GI obstruction DI: Dolasetron: prolonged QT interval and arrhythmias; azole antifungals: inhibition CYP3A isoenzyme |
| Mirtazapine | 1.88 mg/cat PO q48h | DI: Concurrent administration with other MAO inhibitors (i.e., selegiline, amitraz, tramadol, amitriptyline, clomipramine) and/or SSRIs (i.e., fluoxetine) contraindicated DA: Kidney or liver dysfunction |
CRI, Constant rate infusion; hx, history.
Mirtazapine
Recently, the pharmacokinetics and pharmacodynamics of mirtazapine have been reported in cats. In a group of healthy cats, mirtazapine was found to be an effective appetite stimulant, with a shorter half-life than that reported in humans. The recommended oral dose is 1.88 mg/cat every 48 hours.55a In humans, age and kidney and liver dysfunction affect mirtazapine metabolism (hepatic CYP 450 enzymes) and clearance (excreted in urine and feces), suggesting that dose adjustment may be necessary.69a Side effects reported in cats treated with mirtazapine include behavior changes (vocalization and interaction), tremors, muscle twitching, and hyperactivity.9a,55a
Metoclopramide
Metoclopramide is both an antiemetic and prokinetic drug that acts peripherally on the gastrointestinal tract and centrally within the central nervous system (CNS). At low doses metoclopramide inhibits dopaminergic (D2) transmission, and at higher doses it inhibits serotonergic 5-HT3 receptors in the chemoreceptor trigger zone (CRTZ).15,23 Metoclopramide also acts peripherally as a prokinetic at the level of the gastrointestinal smooth muscle of the stomach and duodenum, triggering gastric emptying and duodenal contractions. Multiple mechanisms mediate metoclopramide’s prokinetic activity, including augmentation of acetylcholine release and increased smooth muscle sensitivity to cholinergic neurotransmission, which may in part be because of antagonism of dopamine, but more recently, serotonergic 5HT4 receptor activation has been suggested.23,56 Metoclopramide has been reported to increase the lower esophageal sphincter tone in humans,20 although in cats metoclopramide’s affect on the lower esophageal sphincter is reported to be weak.32
Dopamine is a less important neurotransmitter in the chemoreceptor trigger zone of cats than alpha2-adrenergic and 5-HT3-serotonergic receptors, suggesting that D2-dopaminergic antagonist may be a less effective antiemetic in cats. Clinically metoclopramide commonly controls vomiting in cats, although this clinical response may be secondary to 5-HT3 antagonism and/or its prokinetic effects.32,44
Extrapolated from the short elimination half-life of metoclopramide in dogs (90 minutes), frequent intermittent dosing or delivery by a constant rate infusion (CRI) is necessary. Empirical dosing in cats is 0.2 to 0.4 mg/kg subcutaneously or orally every 8 hours or 1 to 2 mg/kg/day as a CRI. Approximately 25% of metoclopramide is excreted in the urine, thus dose reduction is recommended in cats with underlying renal azotemia.42
Dolasetron and Ondansetron
The clinical use of dolasetron and ondansetron in cats has not been associated with reported side effects, and experimental studies report minimal toxicity in animals at doses 30 times the antiemetic dose.15 Side effects reported in humans include headaches, elevated liver enzymes, rare hypersensitivity reactions, prolongation of the QT interval, and arrhythmias.14,24
Maropitant
Maropitant is a neurokinin-1 (NK-1) receptor antagonist, blocking the binding of substance P to the NK-1 receptors located in the emetic center, CRTZ, and the enteric plexus.55 In cats maropitant has been reported to be efficacious in treating xylazine-induced vomiting and motion sickness.31 Recommended dosing in cats is 1 mg/kg intravenously, subcutaneously or orally every 24 hours for up to 5 days.31 Maropitant is reported to be well tolerated in cats.
Cisapride
Cisapride is a serotonergic 5-HT4 agonist that increases propulsive gastrointestinal motility from the lower esophageal sphincter to the colon. Cisapride binds serotonergic 5-HT4 receptors in the myenteric plexus, increasing the release of acetylcholine in gastrointestinal smooth muscle. In dogs cisapride has greater prokinetic activity in the stomach relative to metoclopramide.29 Cisapride has no direct antiemetic effect, although it is indicated in a vomiting cat with colonic dysmotility secondary to megacolon. Colonic distention can trigger the vomiting reflex in cats. Cisapride induces colonic smooth muscle contractions in cats with megacolon that is dependent on the influx of extracellular calcium and is only partially cholinergic dependent.30 Other potential indications include refractory generalized ileus or gastroesophageal reflux. Dosage recommendations based on the pharmacokinetics in healthy cats is 1.5 mg/kg orally every 12 hours.41 Prior to the use of cisapride, an intestinal obstruction should be ruled out because of its strong prokinetic effects.
Side effects reported in humans are cramping and diarrhea. Potentially life-threatening side effects include QT prolongation and ventricular arrhythmias, the primary concern in humans that led to cisapride’s removal from the market in the United States.47 In cats QT prolongation associated with cisapride administration requires 20 times the therapeutic dose.37 Because of the risk of prolongation of the QT interval and ventricular arrhythmias, the concurrent use of cisapride and dolasetron is not recommended.14 Other potential drug interactions associated with cisapride include concurrent therapy with azole antifungals (ketoconazole and itraconazole), because of their inhibition of hepatic CYP3A isoenzyme system and the inhibition of cisapride metabolism.47
Dietary Modification
Diet trials are commonly used in cats with idiopathic gastrointestinal signs or in cats with suspected or known food hypersensitivities. Dietary strategies used to control vomiting in cats focus on either a highly digestible diet or an elimination (novel protein/carbohydrate or hydrolyzed protein) diet.72 The empirical use of elimination diets in cats is reported to be relatively successful, with approximately 50% of cats with idiopathic gastrointestinal signs responsive to a novel protein/carbohydrate diets within 2 to 3 days.28 Interestingly, traditional diet trials are recommended for a minimum of 8 to 12 weeks, but in this group of diet-responsive cats with chronic gastrointestinal disease, clinical improvement was reported within days.28 Thus if a cat is going to be diet responsive, clinical improvement to a diet trial should be noted relatively early.
Targeted Therapies with Specific Indications for Vomiting
Gastrointestinal Ulcers
See Tables 23-4 and 23-5 for information on gastrointestinal ulcers.
TABLE 23-4 Mechanism of Action and the Adverse Effects of the Common Drugs Used to Treat Gastric Ulcers in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Famotidine (increases gastric pH) | H2 antagonism | |
| Ranitidine (increases gastric pH) (prokinetic) | H2 antagonism Anticholinesterase | Hypotension (IV) |
| Omeprazole (increased gastric pH) | H+/K+ ATPase inhibitor | |
| Sucralfate (gastric ulcer healing) | Prevents H+ back diffusion, inactivates pepsin, absorbs bile acids, and increases gastric mucosal prostaglandin synthesis |
TABLE 23-5 Dosage Recommendations, Contraindications, Potential Drug Interactions, and Clinical Indications for Dosage Adjustments for the Common Drugs Used to Treat Gastric Ulcers in Cats
| Drug | Dosage (Cats) | C: Contraindications DI: Drug Interactions DA: Dosage Adjustments |
|---|---|---|
| Famotidine | 0.5 mg/kg IV, SC, PO q12-24h | DA: azotemia |
| Ranitidine | 2.5 mg/kg IV q12h 3.5 mg/kg PO q12h | DA: azotemia |
| Omeprazole | 0.5-1 mg/kg PO q24h | DI: inhibition CYP2C: diazepam Do not crush enteric coated tablets |
| Sucralfate | 250 mg PO q12h | DI: decreases oral absorption of fluoroquinolones, tetracyclines, and digoxin |
Famotidine
Famotidine has no direct antiemetic effect but is a competitive inhibitor of the histamine (H2) receptors associated with the gastric parietal cells. The H2-receptor is the dominant receptor involved in gastric acid secretion. H2-receptor antagonism is reported to result in a 70% to 90% reduction in acid production.13 Famotidine is more effective at suppressing gastric acid secretion relative to ranitidine. Famotidine is well tolerated, although, with chronic therapy, there is the potential for hypoacidity and gastric bacterial overgrowth. In humans dose reduction is recommended in association with renal dysfunction.21 Famotidine is not an inhibitor of the hepatic microsomal cytochrome P-450 enzyme system, therefore significant drug interactions are not anticipated.
Ranitidine
Ranitidine is also a competitive inhibitor of the H2 receptor associated with gastric parietal cells. In addition, ranitidine increases lower esophageal sphincter tone and functions as a prokinetic agent (increasing gastric emptying and stimulating intestinal motility, including colonic motility), because of its anticholinesterase activity.40,54 Significant drug interactions associated with hepatic microsomal cytochrome P-450 enzyme system inhibition are not a clinical concern with ranitidine.46 An adverse effect to be aware of in cats treated with ranitidine is transient hypotension associated with ranitidine administered as an IV bolus.19 In humans dose reduction is recommended in patients with renal azotemia.39
Ranitidine is effective in decreasing gastric acid in cats.22 Ranitidine would be a logical choice in a cat with gastrointestinal ulceration and/or atony. The reported dosage recommendation for ranitidine in cats is 3.5 mg/kg orally every 12 hours or 2.5 mg/kg intravenous every 12 hours.19
Omeprazole
Omeprazole is a proton pump inhibitor that targets the H+/K+ ATPase pump on the luminal surface of partial cells. Omeprazole is effective at suppressing parietal cell acid secretion, and its effects persist for ≈24 hours after drug withdrawal because of drug accumulation in the parietal cell (by ion trapping). Indications for omeprazole therapy are for the treatment and prevention of nonsteroidal antiinflammatory drug (NSAID)–induced ulcers.9 Omeprazole is enteric coated to prevent its degradation by gastric acid; therefore oral formulations should not be crushed. Based on human studies, omeprazole is a hepatic microsomal cytochrome P-450 enzyme inhibitor with known drug interactions with diazepam.2 The extent of clinically significant drug interactions in cats has yet to be studied.
Omeprazole is reported to be effective in reducing gastric acid secretion in cats.22 The recommended empirical dosage in cats is 0.5 to 1 mg/kg orally once daily. Long-term use in humans33 and dogs11 is associated with gastric polyps and parietal cell hyperplasia, respectively, but the effect of long-term use in cats is currently unknown.
Nonspecific Supportive Therapies for Diarrhea
Dietary Modification
Diet trials are used in some cats with diarrhea if the underlying cause is from known or suspected food hypersensitivities. Dietary management includes either a highly digestible diet, an elimination (novel protein/carbohydrate or hydrolyzed protein) diet (see above for both), or a diet high in fiber.72
High-Fiber Diets
High-fiber diets contain a mixture of both soluble and insoluble fiber that can be beneficial in patients with signs of large bowel diarrhea. Insoluble fiber, such as cellulose, functions to increase the bulk of the stool, bind fluid, and regulate intestinal motility. Soluble fiber, including fruit and vegetable pectins and beet pulp, functions as a source of butyric acid that can be used by the colonic mucosa and decreases proinflammatory cytokines.69,72
Vitamin Supplementation
Cobalamin
Cobalamin (vitamin B12) is an essential vitamin needed by a number of different enzymes, including key enzymes involved in methionine metabolism and the conversion of methylfolate to tetrahydrofolate needed for DNA synthesis. Cobalamin and folate are intimately linked, and hypocobalaminemia can lead to a functional deficiency of folate.57 Ingested cobalamin requires intrinsic factor binding for enterocyte absorption at the level of the ileum.
Hypocobalaminemia is commonly associated with distal small intestine diseases in cats, including inflammatory bowel disease. In addition, low cobalamin has a negative impact on enterocyte function; therefore in many cats with intestinal disease and hypocobalaminemia, cobalamin supplementation is necessary for resolution of clinical signs.60,64 Quantification of serum cobalamin levels is recommended in cats with clinical signs of small bowel diarrhea, ones suspected to have an infiltrative disease of the small intestine (inflammatory bowel disease or gastrointestinal lymphoma), or ones with pancreatic dysfunction. When hypocobalaminemia is identified, supplementation is recommended (250 µg/cat every 7 days) while the underlying cause of cat’s malabsorption is being investigated and at initiation of targeted therapy.
Probiotics and Prebiotics
Probiotics
Probiotics are ingested live microorganisms intended to benefit the host, specifically to support the microflora environment of the gastrointestinal tract as well as to provide an overall benefit to the body’s immune function by immunomodulation.8,18,51 Probiotics chemically modify ingesta and intestinal mucus, as well as affect immune cells, enterocytes, and goblet cells within the intestinal mucosa through direct receptor interactions and indirectly through the action of cytokines.
The microorganisms commonly used are nonpathogenic bacteria and yeast that have a vital role in gastrointestinal health, including Lactobacillus spp., Enterococcus faecium, Bifidobacterium spp., and Saccharomyces spp. For example, lactobacilli synthesize B vitamins, digestive enzymes, and folate coenzymes.63 Clinical indications for the use of probiotics are diverse, including primary gastrointestinal disease, chronic renal disease, and pancreatitis.71
The rational use of probiotics in the treatment of gastrointestinal diseases include their ability to modulate gastrointestinal flora, minimize colonization by pathogenic bacteria, and decrease the likelihood of bacterial translocation.17 In healthy cats, Lactobacillus acidophilus is reported to reduce fecal Clostridium counts.45 When Lactobacillus acidophilus was used adjunctively with antimicrobial therapy, fecal shedding of Campylobacter was reduced in cats with Campylobacter-induced diarrhea relative to cats treated with antimicrobials alone.3 Specifically, in cats with gastrointestinal disease, available research supports the probiotic Enterococcus faecium as clinically beneficial in resolving diarrhea in kittens.16 Relative to the control group, the kittens treated with probiotics had increased fecal Bifidobacteria and blood IgA concentrations and decreased fecal counts of Clostridium perfringens.
Prebiotics
Prebiotics are dietary supplements used to select for the more beneficial enteric flora, support gastrointestinal function, and prevent the overgrowth of pathogenic bacteria, including Salmonella, Escherichia coli, Clostridium, or Campylobacter. For a food additive to be considered a prebiotic, it must be nondigestible by the gastrointestinal tract (resistant to gastric acidity, gastrointestinal hydrolysis and absorption), yet fermentable by gastrointestinal microflora to short-chain fatty acids to stimulate the growth of “good” intestinal bacterial.72
Prebiotics include nondigestible oligosaccharides—commonly, oligofructose, fructo-oligosaccharides, mannanoligosaccharides, inulin, chicory, and lactosucrose.72 Reports on the use of prebiotics in cats are limited to their use in healthy cats; healthy cats fed fructo-oligosaccharides were reported to have a trend toward an increase in fecal concentrations of Lactobacilli and a decrease in concentration of C. perfringens and E. coli relative to the controls.65 To date no reports are available on the use of prebiotics in cats with gastrointestinal disease.
Probiotics and prebiotics potentially have a supportive role in the treatment of gastrointestinal disease in cats. The important clinical consideration in the use of probiotics as an adjunctive therapy is to ensure the use of live nonpathogenic microorganisms that have been documented to colonize the intestinal tract of cats. Gastrointestinal flora co-evolve with their host. Gastrointestinal microorganism colonization varies among species and within each individual animal. The distribution of fecal microflora for a given individual is considered unique but stable over time.68
Targeted Therapies with Specific Indications for Diarrhea
Antimicrobials and Antiparasitics
Antimicrobial and antiparasitic therapies for the treatment of feline diarrhea are indicated based on the specific diagnosis of infectious diarrhea, bacterial enteritis, or as adjunctive therapy for inflammatory bowel disease. Infectious pathogens more commonly associated with feline diarrhea include bacterial enteropathies (Clostridium, Campylobacter), protozoal enteropathies (Tritrichomonas foetus, Giardia spp.), and helminthic enteropathies associated with ascarids, hookworms, whipworms, and tapeworms. Only the more common anthelminthic, antimicrobial, and antiprotozoal therapies are discussed below (Tables 23-6 and 23-7). More information about antimicrobials and antiparasitics is found under specific infections in the discussions of Infectious Enteritis and Gastrointestinal Parasites.
TABLE 23-6 Mechanism of Action and Adverse Effects of the Common Antimicrobials and Antiparasitics Used to Treat Specific Causes of Diarrhea in Cats
| Drug | Mechanism of Action | Adverse Effects |
|---|---|---|
| Fenbendazole (anthelmintic) | Binds microtubule beta-tubulin subunits preventing polymerization | |
| Pyrantel pamoate (anthelmintic) | Targets nicotinic acetylcholine receptors of parasites: depolarization and spastic paralysis | |
| Metronidazole (antimicrobial) | Anaerobic environment: converted to unstable intermediates that disrupt bacterial DNA synthesis | |
| Ronidazole (antimicrobial) | Anaerobic environment: converted to unstable intermediates that disrupt bacterial DNA synthesis |
TABLE 23-7 Dosage Recommendations and Spectrum of Activity of the Common Antimicrobial and Antiparasitic Drugs Used to Treat Specific Causes of Diarrhea in Cats
| Drug | Dosage (Cats) | Spectrum |
|---|---|---|
| Fenbendazole | 50 mg/kg PO every 24h × 5 days | Ascarids, hookworms, whipworms, Taenia pisiformis |
| Pyrantel pamoate | 5 mg/kg PO once, repeat in 3 weeks | Ascarids, hookworms, Physaloptera |
| Metronidazole Metronidazole benzoate | 10-15 mg/kg/day 20 mg/kg/day | Anaerobes, Giardia spp. |
| Ronidazole | 30 mg/kg PO q24h | T. foetus |
Metronidazole
Metronidazole is a nitroimidazole antibiotic with an anaerobic antibacterial spectrum with antiprotozoal activity against Giardia spp. In an anaerobic environment, metronidazole is converted to unstable intermediates (nitroso free radicals) that disrupt bacterial DNA synthesis. Immunomodulatory properties capable of inhibiting cell-mediated immunity have been described for metronidazole, although its immunomodulatory properties are reported at dosages well beyond what is recommended for clinical use,62 raising questions about the clinical use of metronidazole as an adjunctive therapy for treating inflammatory bowel disease.34,43
Resistance to metronidazole is considered rare.43 The most common adverse reaction is gastrointestinal upset, including inappetence, anorexia, nausea, and vomiting. Profuse salivation can occur in cats after oral administration of metronidazole base (formulation used in standard tablets), which has lead to the use of metronidazole benzoate (a compounded formulation not approved by the Food and Drug Administration) in some cats because of its better oral palatability.61 At high doses (>200 mg/kg/day) benzoic acid is reported to be neurotoxic in cats, but with appropriate clinical dosing of metronidazole benzoate benzoic acid toxicity is unlikely.6 Dose-related metronidazole toxicity in cats results in cerebellovestibular ataxia secondary to gamma-aminobutyric acid (GABA) inhibition at dosages greater than or equal to 58 mg/kg/day12,52; clinical signs include nystagmus, head tilt, ataxia, seizures, and obtundation.
In cats with inflammatory bowel disease, the dosage recommendation for the metronidazole base is 10 to 15 mg/kg/day. Metronidazole benzoate contains approximately 60% metronidazole base by weight, translating to an empirical dosage of 20 mg/kg/day of metronidazole benzoate (equivalent to 12.4 mg/kg/day of metronidazole base).61 Little is known about the safety of chronic metronidazole use in cats, but oral metronidazole has been reported to disrupt DNA within feline peripheral mononuclear cells following 7 days of therapy.61 This metronidazole-induced genotoxicity is reversible and is no longer detected 6 days after antibiotic therapy is discontinued.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree