CHAPTER 9 Dietary Therapy of Diseases of the Lower Urinary Tract
INCIDENCE
Lower urinary tract disease occurs commonly in cats. Previous estimates of the incidence in the United States and United Kingdom were 0.85 to 1.0 per cent per year.1,2 These estimates were based on presence of clinical signs only and therefore did not consider actual diagnoses. The proportional morbidity rate, defined as the frequency with which cases are seen at veterinary hospitals, has been reported to be 10 per cent3; although 1 to 6 per cent is reported more commonly.3,4 In a cross-sectional study of 15,226 cats examined at 52 private practices, cats were likely to be examined because of renal disease, cystitis, feline urologic syndrome, and inappetence.5
FELINE LOWER URINARY TRACT DISEASE
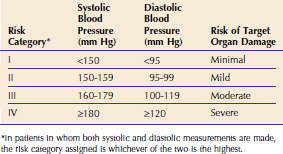
Any disorder of the lower urinary tract may cause signs of lower urinary tract disease. In two prospective studies, one of 143 cats6 and one of 109 cats7 with lower urinary tract disease, idiopathic cystitis was diagnosed most commonly (Figure 9-1). In a retrospective study performed at the University of Georgia and the University of Tennessee, of 110 cats more than 10 years of age with lower urinary tract disease, bacterial cystitis was diagnosed most commonly.8 Thus, causes of lower urinary tract disease appear to be different in different-aged cats, although clinical signs are similar.
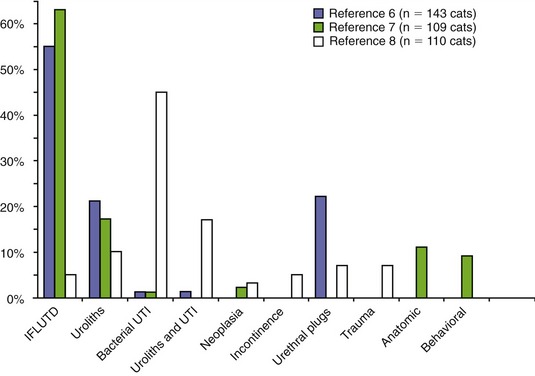
Figure 9-1 Percentage of cats evaluated with lower urinary tract disease in two prospective6,7 and one retrospective8 studies. Cats in the retrospective study were greater than 10 years of age.
IDIOPATHIC CYSTITIS
The role of canned diet in managing cats with idiopathic cystitis has been evaluated in two studies. In one nonrandomized, prospective study of cats with idiopathic cystitis, recurrence of clinical signs occurred in 11 per cent of cats consuming a canned food and in 39 per cent of cats consuming a dry food.9 The diets evaluated in this study were acidifying and formulated to prevent struvite crystalluria and urolithiasis. In another study, clinical improvement and decreased recurrence of clinical signs in cats with idiopathic cystitis were associated with the owners feeding canned foods.10 The outcomes of these studies have resulted in the recommendation to feed canned food to cats with idiopathic cystitis; however, these studies were not randomized, controlled trials. Furthermore, specific dietary ingredients have not been evaluated in cats with idiopathic cystitis.
CRYSTAL-RELATED LOWER URINARY TRACT DISEASE
Of the various causes of feline lower urinary tract disease, crystal-related disease accounts for 15 to 45 per cent of cases. There are many minerals that may precipitate in the urinary tract to form crystals and stones; however, more than 90 per cent of uroliths from cats are composed either of struvite (magnesium ammonium phosphate hexahydrate) or of calcium oxalate monohydrate or dihydrate (Figure 9-2). Struvite is the most common mineral observed to occur in matrix-crystalline urethral plugs (Figure 9-3).
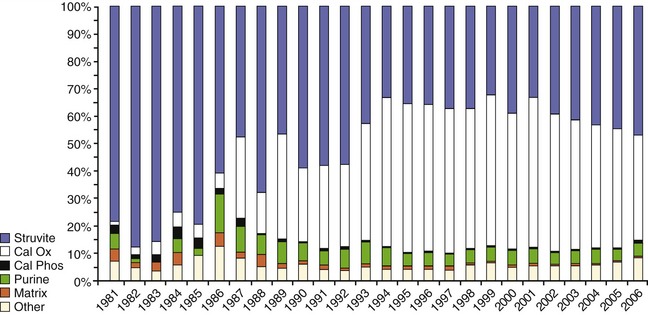
Figure 9-2 Mineral composition of 55,418 uroliths retrieved from cats and analyzed at the Minnesota Urolith Center between 1981 and 2006.61 Cal Ox, Calcium oxalate, monohydrate, and/or dihydrate; Cal Phos, calcium phosphate.
Figure 9-3 Mineral composition of 1901 matrix-crystalline urethral plugs retrieved from male cats and analyzed at the Minnesota Urolith Center between 1981 and 1997.57
Urolith and matrix-crystalline plug formation involves complex physiochemical processes. Major factors include (1) urine supersaturation resulting in crystal formation (nucleation); (2) effect of inhibitors of mineral nucleation, crystal aggregation, and crystal growth; (3) crystalloid complexors; (4) effects of promoters of crystal aggregation and growth; (5) effects of noncrystalline matrix; and (6) urine retention or slowed transit for the process to occur.11,12 Urethral matrix-crystalline plugs have only been identified in male cats and may represent an intermediate phase between lower urinary tract inflammation without crystals and urolith formation.13 The most important driving force behind urolith formation is urinary supersaturation with calculogenic substances14; however, as mentioned before, other factors are important. The goal of treatment of urinary crystal-related disease is to promote a reduced state of urinary saturation.
Urolithiasis
Calcium Oxalate
Calcium oxalate urolith formation occurs when urine is oversaturated with calcium and oxalate.14 In addition to these alterations in activities of ions, high-molecular-weight proteins occurring in urine, such as nephrocalcin, uropontin, and Tamm-Horsfall mucoprotein, influence calcium oxalate formation.15 We have a limited understanding of the role of these macromolecular and ionic inhibitors of calcium oxalate formation in cats. Certain metabolic factors are known to increase risk of calcium oxalate urolith formation in several species, including cats. Medical and nutritional strategies for stone prevention have focused on amelioration of these factors.
Hypercalcemia is associated with increased risk of calcium oxalate urolith formation. In cats with calcium oxalate uroliths, hypercalcemia was observed in 35 per cent of the cases.16 Conversely, uroliths developed in 35 per cent of cats with idiopathic hypercalcemia.17 Hypercalcemia, when severe, results in increased calcium fractional excretion and hypercalciuria.
Hypercalciuria is a significant risk factor but not necessarily the cause of calcium oxalate urolith formation in human beings, dogs, and cats.18 Hypercalciuria can result from excessive intestinal absorption of calcium (gastrointestinal hyperabsorption), impaired renal reabsorption of calcium (renal leak), and/or excessive skeletal mobilization of calcium (resorptive).12 In Miniature Schnauzers, gastrointestinal hyperabsorption appears to occur most commonly, although renal leak hypercalciuria also has been observed.19 Hypercalciuria has not been well defined in normocalcemic cats with calcium oxalate uroliths but is thought to occur.
Metabolic acidosis promotes hypercalciuria by promoting bone turnover (release of calcium with buffers from bone), increasing serum ionized calcium concentration (resulting in increased urinary calcium excretion), and decreasing renal tubular reabsorption of calcium. In cats consumption of diets supplemented with the urinary acidifier ammonium chloride has been associated with increased urinary calcium excretion.20 Significant aciduria (urine pH < 6.2) may represent a risk factor for calcium oxalate formation because of acidemia and hypercalciuria. In addition, acidic urine alters function and concentration of crystal inhibitors. Low urine pH decreases urinary citrate concentration by increasing renal proximal tubular citrate reabsorption. Acidic urine is known to impair function of macromolecular protein inhibitors.
Oxalic acid is a metabolic end product of ascorbic acid (vitamin C) and several amino acids, such as glycine and serine, derived from dietary sources. Oxalic acid forms soluble salts with sodium and potassium ions but a relatively insoluble salt with calcium ions. Therefore any increased urinary concentration of oxalic acid may promote calcium oxalate formation. Dietary increases of oxalate and vitamin B6 deficiency are known factors increasing urinary oxalate. Hyperoxaluria has been observed experimentally in kittens consuming vitamin B6–deficient diets11 but has not been associated with naturally occurring calcium oxalate urolith formation. Genetic anomalies also may increase urine oxalic acid concentration. Hyperoxaluria also has been recognized in a group of related cats with reduced quantities of hepatic D-glycerate dehydrogenase, an enzyme involved in metabolism of oxalic acid precursors (primary hyperoxaluria type II).21 Hyperoxaluria also has been associated with defective peroxisomal alanine/glyoxylate aminotransferase activity (primary hyperoxaluria type I) and intestinal disease in human beings (enteric hyperoxaluria). These abnormalities have not been evaluated in cats.
Decreased urine volume results in increased calcium and oxalic acid saturation and an increased risk for urolith formation. Cats can achieve urine specific gravities in excess of 1.065, indicating a marked ability to produce concentrated urine. Many cats affected with calcium oxalate uroliths have a urine specific gravity greater than 1.040 unless there is some impairment of renal function or urine-concentrating ability.18
Detection of calcium oxalate crystals indicates that urine is supersaturated with calcium oxalate and, if persistent, represents an increased risk for calcium oxalate urolith formation. However, calcium oxalate crystalluria is present in less than 50 per cent of feline cases at time of diagnosis of calcium oxalate urolithiasis.18
Medical protocols that will promote dissolution of calcium oxalate uroliths currently are not available; therefore uroliths must be removed physically, either surgically or by voiding urohydropropulsion22 (see Chapter 52).
Nutritional and/or medical protocols should be considered to minimize urolith recurrence or to prevent further growth of uroliths remaining in the urinary tract. A significant number of cats will develop recurrent uroliths within 2 years of their initial episode if prevention protocols are not initiated.23 If possible, metabolic factors known to increase calcium oxalate risk should be corrected or minimized. Goals of dietary prevention include (1) reducing urine calcium and oxalate concentration, (2) promoting high concentrations and activity of urolith inhibitors, (3) reducing urine acidity (increase urine pH), and (4) promoting dilute urine.
Increasing urine volume is a mainstay of preventive therapy for calcium oxalate urolithiasis in human beings. By increasing water intake, urinary concentrations of calculogenic minerals are reduced. In addition, larger urine volumes typically increase urine transit time and voiding frequency, thereby reducing retention time for crystal formation and growth. Feeding cats a canned food is the most practical means of increasing water intake and lowering calcium oxalate urine saturation. The goal is to dilute urine to a specific gravity of less than 1.030.24 Flavoring water, enhancing water access, and adding water to dry foods may be helpful for cats who refuse to eat canned foods. Sodium chloride should not be added routinely to the diet to stimulate thirst. Although cats will increase water intake and dilute urine in response to salt, the consequence of high-sodium foods in cats prone to oxalate uroliths is unknown. Increased dietary sodium may increase urinary calcium excretion in at-risk cats and can contribute to ongoing renal damage in cats with marginal renal function.24
The solubility of calcium oxalate in urine is influenced minimally by pH; however, epidemiological studies consistently identify acidifying diets among the most prominent risk factors for calcium oxalate urolithiasis.25–27 Persistent aciduria may be associated with low-grade metabolic acidosis, which promotes bone mobilization and increases urinary calcium excretion. In a case series of five cats with hypercalcemia and calcium oxalate uroliths, discontinuation of acidifying diets or urinary acidifiers was associated with normalization of serum calcium concentration.26 Furthermore, aciduria promotes hypocitraturia and functional impairment of endogenous urolith inhibitors. Thus, feeding an acidifying diet or administering urinary acidifiers to cats at risk for calcium oxalate is contraindicated. A target urine pH of 6.6 to 7.5 is suggested in cats at risk for calcium oxalate urolith recurrence.24
Although reduction of urine calcium and oxalic acid concentrations by restriction of dietary calcium and oxalic acid appears logical, it is not without risk. Reducing consumption of only one of these constituents may increase availability and intestinal absorption of the other, resulting in increased urinary excretion. Conversely, increasing dietary calcium levels in normal cats contributes directly to increased urine calcium concentration. Because epidemiological data in cats suggest that marked dietary calcium restriction increases urolith risk, moderate levels of dietary calcium are advised in nonhypercalcemic cats.24
Urinary oxalate is derived from endogenous metabolism of oxalate precursors (i.e., glycine and ascorbic acid) and dietary oxalic acid. Most pet food ingredients are low in oxalic acid, with the exception of vegetables, legumes, and several vegetable-based fermentable fibers (e.g., beet pulp and soybean fiber). Dietary oxalic acid concentrations in foods for cats should be reduced to the lowest possible level. Suggested levels are less than 20 mg oxalic acid/100g of food (dry matter basis).25
Excess intake of vitamin C, a metabolic oxalate precursor, similarly should be avoided.24 Although normal dietary vitamin C levels are not considered a risk in human beings, very small increases in urinary oxalate are a concern in urolith formers. Because cats do not have a dietary vitamin C requirement, supplementation should be avoided in foods fed to cats at risk for calcium oxalate uroliths. Cranberry concentrate tablets also are contraindicated. They provide mild acidification and are high in oxalate, as well as vitamin C.28
Potassium citrate often is included in diets designed for calcium oxalate prevention. In urine, citric acid combines with calcium to form soluble complexes, thereby reducing ionic calcium concentration. Citric acid also inhibits nucleation of calcium and oxalate crystals directly. When oxidized within the tricarboxylic acid cycle, supplemental citrate results in urine alkalinization due to production of bicarbonate. The metabolic alkalinization increases endogenous renal citrate excretion and reduces calcium absorption and urinary excretion.24 Commercial products that add citrate but continue to acidify the urine (pH < 6.5) negate the benefit of citrate therapy.
Consumption of high levels of sodium may augment renal calcium excretion in human beings. Recent studies in healthy cats did not find increased urine calcium excretion in response to high dietary salt intake.24 In cats with marginal renal function and increased calciuria, sodium exacerbated calcium excretion. No studies have evaluated the effect of sodium in cats prone to calcium oxalate stones. Epidemiological evidence suggests that low dietary sodium levels in cat foods increase the risk for calcium oxalate urolithiasis.29 Nonetheless, when fed a food lower in sodium, cats with naturally occurring calcium oxalate uroliths excreted less urine calcium.23 Until further data are available, treatments for diuresis that include orally administered sodium chloride or loop diuretics, which promote renal sodium excretion, should be used cautiously and with careful monitoring because they may increase risk of calcium oxalate urolith formation in some patients. Recommended levels of sodium in foods for cats predisposed to calcium oxalate formation is between 0.3 and 0.5 per cent sodium on a dry matter basis.
Dietary phosphorus should not be restricted in cats with calcium oxalate urolithiasis. Low dietary phosphorus is a risk factor for calcium oxalate urolith formation in cats.29 Reduction in dietary phosphorus may be associated with activation of vitamin D, which in turn promotes intestinal calcium absorption and hypercalciuria. Additionally, phosphate status determines pyrophosphate urinary concentrations, an inhibitor of calcium oxalate urolith formation in human beings and rodents. If calcium oxalate urolithiasis is associated with hypophosphatemia and normal calcium concentration, oral phosphorus supplementation may be considered. Caution should be used, however, because excessive dietary phosphorus may predispose to formation of calcium phosphate uroliths. Whether this occurs in cats is unknown. Phosphorus levels in the foods for cats predisposed to calcium oxalate formation should not be excessive. Levels from 0.5 to 0.8 per cent have been recommended.24
Urinary magnesium forms complexes with oxalic acid, reducing the amount of oxalic acid available to form calcium oxalate. Studies in cats associate low dietary magnesium with calcium oxalate risk.26,27,29–32 In human beings, supplemental magnesium has been used to minimize recurrence of calcium oxalate uroliths; however, supplemental magnesium may increase the risk of struvite formation in cats. At this time, because the risks and benefits of magnesium supplementation to cats with calcium oxalate urolithiasis have not been evaluated, it is not advised. It appears logical that magnesium should not be highly restricted in diets that are consumed by cats with calcium oxalate urolithiasis. Many diets that claim to benefit feline “urinary tract health” are reduced in magnesium and promote urinary acidification. These foods are designed for struvite prevention and are not appropriate for cats at risk for calcium oxalate urolithiasis. Prudent levels of dietary magnesium are from 0.08 to 0.10 per cent dry matter, or approximately 20 mg magnesium/100 kcal.24,29
Consumption of high amounts of animal protein by human beings is associated with an increased risk of calcium oxalate formation. Dietary protein of animal origin may increase urinary calcium and oxalic acid excretion, decrease urinary citrate excretion, and promote bone mobilization in order to buffer the acid intake from metabolism of animal proteins. However, a case control study showed that higher protein concentration in cat foods appeared protective against calcium oxalate uroliths.29 Protein levels between 8 and 9 g protein/100 kcal appeared most protective. Although several coassociations (e.g., higher protein in canned foods) might explain this finding, cats are obligatory carnivores, and dietary protein restriction in the management of calcium oxalate urolithiasis is not advised.
The diet should be adequately fortified with vitamin B6, because vitamin B6 deficiency promotes endogenous production and subsequent urinary excretion of oxalic acid.33 There is no evidence that providing increased vitamin B6 beyond meeting the nutritional requirement provides a benefit in cats. Because most commercial diets designed for cats are well fortified with vitamin B6, it is unlikely that additional supplementation will be beneficial, except in homemade diets. Regardless, vitamin B6 is reasonably safe and sometimes is provided to cats with persistent calcium oxalate crystalluria or frequent recurrences.
Increased dietary fiber intake is associated with decreased risk of calcium oxalate recurrence in some human beings, but not in cats unless they are hypercalcemic. Certain types of fiber (soy or rice bran) decrease calcium absorption from the gastrointestinal tract, which may decrease urinary calcium excretion. Also, higher-fiber diets tend to be less acidifying. In five cats with idiopathic hypercalcemia and calcium oxalate uroliths, feeding a high-fiber diet with supplemental potassium citrate resulted in normalization of serum calcium concentrations34; however, efficacy of increased fiber intake is unproved at this time.
At the time of writing, four therapeutic foods are formulated and marketed for the prevention of calcium oxalate uroliths in cats (Table 9-1). These diets either contain potassium citrate (as an alkalinizing agent and as a source of citrate) and are designed to induce a higher urine pH than standard foods and limit mineral intake, or they are designed to promote significant increases in water intake. Consumption of Prescription Diet Feline c/d Multicare (Hill’s Pet Nutrition, Inc., Topeka, KS) and Urinary SO (Royal Canin, St. Charles, MO) by healthy cats results in low urine saturation with calcium oxalate. Clinical trials using the predecessor to c/d Multicare (Prescription Diet Feline x/d) in cats with naturally occurring calcium oxalate urolithiasis reduced calcium oxalate supersaturation by 59 per cent.23 The reduction in calcium oxalate formation product appeared to be a function of its ability to lower urine calcium. We have had some success in reducing mild hypercalcemia in certain calcium oxalate urolith–forming cats by feeding a high-fiber diet (Prescription Diet Feline w/d; Hill’s Pet Nutrition Inc., Topeka, KS) and by administering potassium citrate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree