CHAPTER 40 Diastolic Dysfunction in Feline Cardiomyopathies
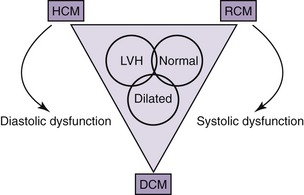
Cardiomyopathies are a significant cause of morbidity and mortality in the feline population. Cardiomyopathy refers to a group of diseases in which the predominant feature is a structural and functional impairment of the heart muscle. These diseases are distinctive because they are not associated with pericardial, hypertensive, congenital, valvular, ischemic, or metabolic causes. Improvements in diagnostic techniques have increased the awareness of primary cardiomyopathy in the feline population and allowed for a more accurate description of the changes in the structure and function of the heart muscle.1–8 Classification of cardiomyopathies has been difficult because of the overlapping nature of many cases that do not fit into one of the major groups. Both morphological and structural classifications are attempted based on information obtained from the two-dimensional, M-mode, and Doppler echocardiograms. The major morphological classifications include hypertrophic, restrictive, dilated, and right ventricular cardiomyopathy.9 Despite the popularity of the morphological descriptions in clinical practice, an attempt to identify the underlying physiological consequences should be made. Morphological myocardial changes result in alterations of systolic function, diastolic function, or both (Figure 40-1). An understanding of the physiological implications of the disease ultimately will allow for more focused therapy. Publications continue to emerge on diastolic dysfunction in normal cats and in cats with heart disease.
Diastolic dysfunction is the primary functional disturbance in hypertrophic and restrictive cardiomyopathies.10,11 Physical examination, electrocardiography (ECG), and thoracic radiographs are unreliable in the diagnosis of diastolic dysfunction. Accurate assessment of diastolic function previously required the use of more invasive testing, including measurements of cardiac pressures, rate of left ventricular relaxation, and left ventricular compliance.12,13 Doppler echocardiography is a noninvasive emerging method in feline cardiology to allow earlier and more accurate estimation of diastolic function.14–19 Quantification of diastolic dysfunction will provide us with a baseline to be able to assess therapeutic efficacy in future clinical trials. Ideally, therapy would target a specific physiological process and the response to therapy measured quantitatively through long-term follow-up echocardiographic data.20,21
Assessment of hemodynamic properties requires basic understanding of cardiac physiology, including diastolic properties and left ventricular filling patterns. This chapter serves as an introduction to the concept of diastology by discussing the definition, physiology, diagnostic testing, diseases, and potential therapeutic options. A detailed description of feline cardiomyopathies, including clinical presentation, diagnosis, and therapy, can be found in Chapters 33 and 34 of the fifth volume of this series.
DEFINITION
Diastolic dysfunction is defined as the inability to fill the left ventricle to a normal end-diastolic volume without an abnormal increase in left ventricular end-diastolic or mean left atrial pressure. A failure to increase left ventricular end-diastolic volume resulting in an elevated filling pressure leads to reduced exercise capacity and signs of pulmonary congestion.22–24
PHYSIOLOGY
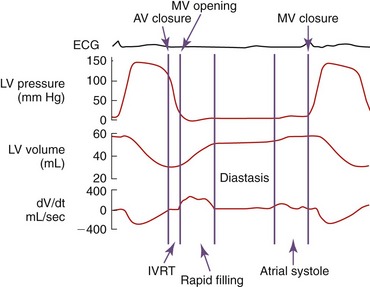
Diastole is the period of the cardiac cycle defined as beginning with closure of the aortic valve and ending with closure of the mitral valve. Ventricular relaxation begins at approximately the time of peak ventricular pressure and is manifested as a reduction in left ventricular pressure. Diastole is divided into four phases: (1) isovolumic relaxation (IVR), (2) early diastolic filling, (3) diastasis (mid-diastole), and (4) atrial contraction (Figure 40-2). The first phase, IVR, begins when the left ventricular pressure falls below the aortic pressure, resulting in cessation of forward aortic flow and closure of the aortic valve. During this phase the left ventricular volume remains constant as left ventricular pressure continues to decline. Geometrical changes to the left ventricle continue during this period. The isovolumic phase ends when the left ventricular pressure falls below left atrial pressure, the mitral valve opens, and ventricular filling begins. During the second phase, early diastolic filling, blood enters the left ventricle from the left atrium down a pressure gradient generated by continued ventricular relaxation. Although the act of relaxation is an active process, blood fills the ventricle passively along its gradient. Negative pressure can be generated during relaxation in the left ventricle, creating a “sucking” effect. Under normal conditions this phase represents the most rapid period of ventricular filling. Because of a reduction in ventricular relaxation, ventricular filling, and atrial emptying, the atrioventricular pressure gradient decreases. When left ventricular and atrial pressure equilibrate, ventricular filling stops. The third phase, diastasis, is the phase in diastole when atrial and ventricular pressures are in equilibrium and little or no filling occurs. Flow from the pulmonary veins may continue to contribute slightly to ventricular filling. The duration of this period is affected by heart rate and can be abolished at higher heart rates. The final phase, atrial contraction, often is defined as the late filling phase. In normal conditions atrial contraction results in a second phase of ventricular filling. During this phase under normal conditions, almost all of the blood flows forward into the left ventricle and little into the pulmonary veins. Left atrial contraction contributes approximately 10 to 30 per cent of the total ventricular filling volume and is affected by many arrhythmias and disease.23
DETERMINANTS OF DIASTOLIC FUNCTION
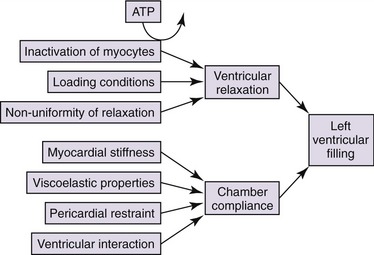
Determinants of left ventricular diastolic performance are affected by systolic performance, heart rate, and cardiac conduction. Two major diastolic properties are left ventricular relaxation and left ventricular compliance (Figure 40-3). In early diastole the rate of ventricular filling is related to the pressure difference between the left atrium and left ventricle when the mitral valve opens. This pressure gradient is mostly determined by the left atrial pressure and the rate of active decline in the left ventricular pressure (ventricular relaxation). Left ventricular relaxation is an active process that requires energy expenditure within the myocardial cells. The active process of relaxation is mostly completed by mid-diastole (diastasis). During the later portion of diastole, the passive properties of the myocardium predominate (myocardial compliance). The transmitral gradient (TMG), which determines the left ventricular filling pattern, is influenced by many factors including both left ventricular relaxation and compliance.24 Faster left ventricular relaxation results in a larger early TMG, more filling in early diastole, and less filling in late diastole. Slower left ventricular relaxation results in a lower early TMG and proportionally less early diastolic filling compared with late diastolic filling. For the same rate of left ventricular relaxation, decreased left ventricular compliance and increased left atrial pressure have the opposite effect on the TMG and can offset a slower rate of relaxation. Changes in rates of left ventricular relaxation and left atrial pressure are a continuum resulting in many different filling patterns for given situations. Therefore the same filling pattern may occur with different combinations of increased left atrial pressure and left ventricular relaxation.24
PATHOGENESIS OF DIASTOLIC DYSFUNCTION
HYPERTROPHIC OBSTRUCTIVE/NONOBSTRUCTIVE CARDIOMYOPATHY
Hypertrophic cardiomyopathy is a disease characterized by abnormal concentric or asymmetric left ventricular hypertrophy.2 Secondary causes of hypertrophy including acromegaly, hypertension, aortic stenosis, and hyperthyroidism should be excluded prior to making the definitive diagnosis. The left ventricle is nondilated with a broad variation in phenotypic changes. The entire left ventricle can be hypertrophied or it may be isolated to a particular wall segment. The hypertrophy can be mild to severe. No single pattern is “classic” or characteristic for the disease. Systolic anterior mitral valve motion (SAM) resulting in a dynamic left ventricular outflow tract obstruction is commonly associated with interventricular septal hypertrophy. The mitral valve is displaced anteriorly during systole, resulting in a pressure gradient midway through systole across the left ventricular outflow tract and a corresponding posteriorly directed mitral regurgitation. The left ventricle typically is hyperdynamic owing to the increased muscle mass.
The primary physiological consequences of ventricular hypertrophy are an elevated left ventricular end-diastolic pressure with a normal or reduced left ventricular end-diastolic volume. These changes result from a stiff and noncompliant ventricle along with alterations in early diastolic filling or ventricular relaxation. Compliance is affected as a result of the increased muscle mass, increased amount of myocardial fibrosis, and disorganization of the ventricular myocytes (see Chapter 43). The presence of a dynamic outflow tract obstruction secondary to SAM results in increased left ventricular systolic pressures, myocardial wall stress, and impedes left ventricular ejection. The left atrium dilates in response to the elevated left ventricular filling pressure, leading eventually to elevated pulmonary venous pressures and congestive heart failure.25,26
RESTRICTIVE CARDIOMYOPATHY
Restrictive cardiomyopathy has been used to morphologically describe nonhypertrophied ventricles with normal systolic function. A presumptive diagnosis of restrictive cardiomyopathy is made based on morphology, but further verification of diastolic function is essential to making a more accurate diagnosis. Left ventricular chamber dimensions usually are normal or mildly reduced, systolic function is normal, and wall thickness is normal to mildly increased. Regional areas of ventricular thinning and scarring may represent myocardial atrophy or infarction. Focal or diffuse areas of hyperechoic and thickened endocardium or wall segments are associated with excessive scarring and fibrous tissue. Fibrous bands of tissue may be seen adjacent to myocardial walls, bridging areas of myocardial thinning, or in some cases bridging the septum, papillary muscles, and free wall obliterating the left ventricular apex (see Chapter 43).4
The physiological consequences of restrictive cardiomyopathy are alterations in the diastolic filling properties of the left ventricle. Changes in ventricular relaxation and chamber compliance are the primary alterations. Elevation in left ventricular end-diastolic pressure with normal or mildly elevated end-systolic volumes result in progressive increases in left atrial pressure. Characteristic mitral inflow velocity profiles have been used to imply restrictive ventricular filling. Several Doppler echocardiographic variables can be used together to determine the extent of diastolic functional impairment.27
DILATED CARDIOMYOPATHY
Reduction in left ventricular systolic function leads to elevation in end-diastolic pressures caused by reduced systolic emptying and increased residual end-systolic volumes. A reduction in cardiac output leads to clinical signs of pulmonary congestion, pleural and abdominal effusions, and in some cases cardiogenic shock.28
ASSESSMENT OF DIASTOLIC FUNCTION
ISOVOLUMIC RELAXATION TIME
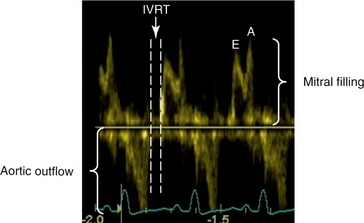
Isovolumic relaxation time (IVRT) represents the time during left ventricular relaxation between closure of the aortic valve and opening of the mitral valve. The IVRT can be readily measured by spectral Doppler echocardiography (Figure 40-4). Simultaneous mitral inflow and aortic outflow tract velocity signals are obtained. The aortic closure and mitral opening interval can be determined.23,24 Normal IVRT has been reported as 71 ± 17 ms in normal anesthetized cats, and 46.2 ± 7.6 ms in conscious cats.29,30 When compared with invasive measures of diastolic function, IVRT was well correlated and a good indicator of diastolic function in anesthetized cats.29 IVRT is prolonged in early diastolic dysfunction (relaxation) and shortened with restrictive cardiomyopathy, because the markedly elevated left atrial pressures result in early opening of the mitral valve. In cats with hypertrophic cardiomyopathy, IVRT was shown to be prolonged.31,32 As with many other Doppler indices of diastolic function, IVRT has been shown to be correlated with age in cats. In a study comparing different age ranges of conscious cats, there was a progressive increase in the IVRT with age.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree