Renaud Léguillette
Diagnostic Procedures for Evaluating Lower Airway Disease
A diagnosis of lower airway pathology cannot be made solely on the basis of results obtained from a diagnostic procedure. Diagnosis must take into account the history, clinical signs, and results from the physical examination, including thorough lung auscultation with a rebreathing bag. For example, cytology results from a bronchoalveolar lavage (BAL) that reveal inflammation cannot be used to diagnose recurrent airway obstruction (RAO) or inflammatory airway disease (IAD) in the absence of history or clinical signs because these conditions, by definition, are identified by a combination of clinical signs and ancillary test results. A thorough history and physical examination gives clinicians a greater feel for the likelihood of lower airway pathologies in horses and can help determine which diagnostic procedures to use according to the sensitivity and specificity of each test. Although the sensitivity and specificity of lower airway diagnostic tests are documented for a few procedures, decisions appear to rely more on experience and knowledge than prediction. Procedures for diagnosing lower airway pathologies can be categorized into imaging, sampling, or physiologic tests.
Endoscopy and Scoring
The lower airways and lungs are difficult to evaluate visually in adult horses because these structures lie in the middle of a large chest cavity. However, it has become routine to perform endoscopy of the airways or thoracic ultrasound in horses now that equipment is portable and affordable.
Endoscopy of the trachea, tracheal septum, and bronchi provides valuable information on the degree of mucus accumulation and inflammation and is used to identify bleeding, masses, foreign bodies, and even lungworm infection. An endoscopic examination alone cannot be used to reach a particular diagnosis because there are no idiosyncratic endoscopic findings for lower airway pathology. However, lower airway endoscopy is a sensitive test for detection of inflammation and potentially infection in the lower airways. Results from upper and lower airway endoscopy should be interpreted independently, because the “one airway, one disease” concept does not seem to apply to horses in terms of generalized airway inflammation.
Equipment and Procedure
Most endoscopes available in equine practices are approximately 1.2 meters long, which is a sufficient length to observe the lower part of the trachea and tracheal septum in smaller horses, but not to sample fluid from the deeper airways. Better imaging of the tracheal septum and bronchi is obtained with a 2.2-meter-long endoscope (such as a human colonoscope) or an equine gastroscope, which are now available in portable versions for ambulatory practice. Sedation is often not necessary when a 1.2-meter endoscope is used for a brief examination of the lower airways. Coughing during the endoscopy procedure is to be expected in horses with airway mucus accumulation or inflammation. When a long endoscope is used to examine the tracheal septum and deeper bronchi, neuroleptanalgesia (administration of an α2-receptor agonist with butorphanol, with the butorphanol given at a dosage of 10 mg/450 kg), in addition to instillation of lidocaine (60 to 120 mL of 2% solution diluted to one-third or one-fourth strength, to approximately 0.5% solution) applied to the larynx and airway mucosa through the endoscope, is needed to decrease the cough response and provide local anesthesia of the airways.
Interpretation and Analysis of Information
To ensure accurate interpretation and reporting, clinicians must be familiar with the anatomy of the equine bronchial tree. The horse’s lung branches monopodially, with smaller daughter bronchi branching from a main larger parent bronchus. It is practical to use a two-digit system of nomenclature to label the bronchi in the horse, in which the first digit indicates the main bronchus (right = 1, left = 2) and the second digit indicates the secondary bronchus bifurcation location (e.g., 1.4 refers to the fourth secondary bronchus in the right lung main bronchus).
Scoring systems are available for thickness of the tracheal septum as well as for the degree of mucus and blood accumulation in the trachea and bronchi. The redness of the mucosa is a subjective finding that is difficult to interpret and requires color calibration of the equipment. Other common findings not subjected to a scoring system include the presence of food, ulcers, masses, and nodular cartilaginous protrusions. Tracheal and bronchial mucus accumulation is one of the most common findings, is easy to observe with a shorter endoscope, and has a well-validated scoring system based on a 0 to 5 scale. Mucus accumulation has many clinical implications, and accurate use of the mucus scoring system is essential. For example, a mucus score of 2 or higher is associated with poor performance in Thoroughbred racehorses. In addition, the presence of mucus is correlated with neutrophil accumulation and hence inflammation in the trachea and lower airways. Mucus accumulation can be secondary to mild to severe inflammatory or infectious processes in the lungs, as well as a response to airborne dust. Tracheal septum thickness at the level of the carina is not correlated with, and is not as clinically significant as, the tracheal mucus score. Still, a higher tracheal septum thickness score is consistent with a higher bronchial mucus score and is increased in horses with severe lung inflammation.
Lastly, blood in the trachea of racehorses with exercise-induced pulmonary hemorrhage (EIPH) is also clinically important because it affects performance. Two scoring systems are used (a 0 to 4 and a 0 to 5 scale) to describe the severity of the blood accumulation. Lower airway endoscopy has a high specificity for EIPH, but it is highly dependent on the timing of tracheal examination. To decrease the risk for obtaining false-negative results, endoscopic examination should be performed 30 to 90 minutes after exercise.
Imaging Procedures
Thoracic Ultrasonography
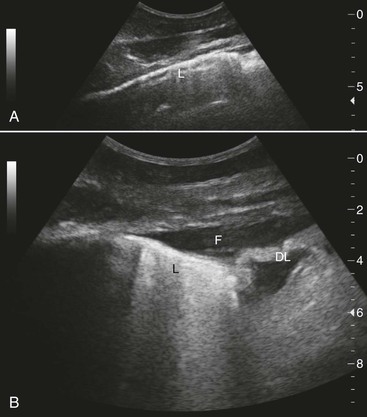
Although it seems counterintuitive to ultrasonographically examine an organ such as the lung that is filled with air, this procedure is diagnostically valuable and more sensitive than chest radiography for detecting disease in the periphery of the lungs. Ultrasound equipment is continuously improving and becoming more affordable. The technique for imaging the lungs is summarized (Box 48-1; Figure 48-1). Caution should be used to avoid overinterpretation of ultrasonography findings. For example, on the right side of the chest in healthy horses, fat commonly deflects the lung interface away from the parietal pleura ventral and caudal to the heart; this should not be interpreted as a sign of pneumonia.
Ultrasonography is ideal for detection and follow-up of horses with pleuropneumonia because of its sensitivity in detecting free fluid and its utility in monitoring development of the chronic cystlike cranial thoracic masses sometimes observed in these cases. In addition, in pneumonia and pleuropneumonia, lung ultrasonography can suggest the presence of anaerobic infection by revealing hyperechoic gas. Ultrasonography can also sensitively detect pneumonia when there is atelectasis or lung consolidation in the lung periphery. In some cases, these abnormalities involve a large area of lung, whereas in others, it is only the presence of superficial craters, comet-tail artifacts (see Figure 48-1), or superficial lung consolidation that indicates lung pathology. Such abnormalities may also occur in horses with influenza. Thoracic ultrasonography can also reveal nonspecific abnormalities like pleural roughening and comet-tail artifacts even with deeper abscesses. Thoracic ultrasonography in M mode can be more sensitive than radiography for detection of pneumothorax. The “seashore and stratosphere” appearance of the interface between free air and collapsed lung is highly sensitive and also enables determination of whether the pneumothorax is in the right or left hemithorax. In racehorses, detection of the comet-tail artifacts is a highly sensitive, but not specific, indicator of EIPH. Neoplasia can be associated with pleural effusion but also with ultrasonographically visible irregularities on the pleural surfaces, for example, in disseminated hemangiosarcoma or granular lung cell tumors. In horses with multinodular pulmonary fibrosis and generalized granulomatous disease, thoracic ultrasound examination will also show nonspecific abnormalities such as hyperechoic nodular lesions on the lung surface. Thoracic ultrasonography also enables specific diagnoses, such as diaphragmatic hernia with small intestine and fluid in the chest cavity; however, in the author’s experience, it is more common to simply find an area of collapsed lung without observing intestinal structures.
Thoracic Radiography
Because of horses’ large size, lung radiography is challenging to both perform and interpret. The distance between horse body surface and screen augments magnification and geometric distortion while dramatically decreasing image resolution. Furthermore, digital radiography systems usually use smaller screens that are not adequately sized for equine thoracic radiography. Orthogonal (dorsoventral) views are not feasible in adult horses, so views should be taken from both the right and the left sides to aid in determining the sagittal plane of a lesion (Box 48-2). Age, size, and respiratory phase should be taken into consideration in interpretation of equine lung radiographs because they affect the radiographic appearance of the lungs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree