Chapter 2 Diagnostic Imaging in the Food Animal
Diagnostic imaging, such as radiographs and ultrasound, is an invaluable and underused tool for the food animal practitioner. Other infrequently used modalities include nuclear medicine, computed tomography (CT), and magnetic resonance imaging (MRI). Because of the variety of available imaging modalities, the proper imaging study must be selected to maximize the chance of detecting lesions while minimizing time and cost to the client. This section will provide an outline for general techniques and indications of when to use various imaging modalities in the food animal.
Radiology
Survey radiography, including digital and computed radiography, is considered the mainstay of diagnostic imaging. The various views that can be acquired have been described in detail in other publications (Bargai et al, 1989; Pharr and Bargai, 1997). A table has been provided to illustrate a general guide—a starting point—for techniques to acquire different radiographic studies in various species (Table 2-1). Unfortunately, the nature of ambulatory work does not allow practitioners the luxury of developing the film, evaluating the radiograph, adjusting the technique, and retaking radiographs if the exposure is unsatisfactory. For this reason, all recommendations assume an average-sized animal and a film-to-tube focal distance of no greater than 40 inches (or as close as possible in regard to thorax and abdomen). If the patient appears thin or obese, decreasing or increasing the kilovolt peak (kVp) by no less than 15% adjusts the technique. Alternatively, the milliamps per second (mAs) can be changed by no less than 50%, depending on the radiographic study required.
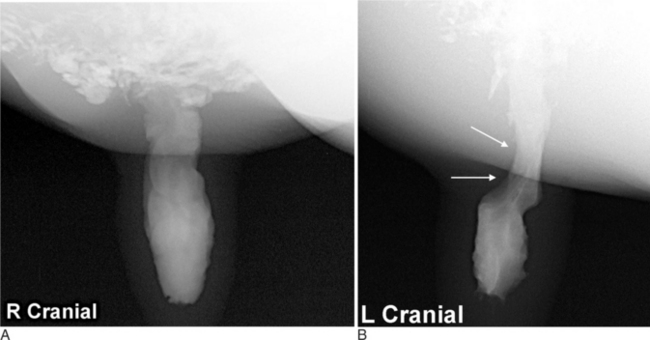
In addition to survey radiographs, contrast radiography can also be performed to provide additional information about soft tissue structures. Contrast procedures that examine the esophagus, digits, mammary gland, and urinary bladder have been described. Positive contrast mammography can be performed on the mammary gland; however, to the author’s knowledge, no milk withdrawal times for iodinated contrast media have been published. In addition, intravenous sodium iodine is not for use in the lactating dairy cow; thus no inferred withdrawal time can be made. Therefore if iodinated contrast media is used in the lactating cow, the recommendation is to strip the teat thoroughly and observe a withdrawal time of approximately 5 days. In the United States, this withdrawal time is only a suggestion, and the authors recommend contacting the local state regulatory commission before performing the procedure. To perform positive contrast mammography, a small volume (10-30 ml) of iodinated contrast media (Hypaque-76*) is infused into the affected and a nonaffected teat via a teat cannula. Lateral radiographic projections are then acquired of each mammary gland separately. Structural lesions of the papillary duct and lactiferous sinus can be detected (Figure 2-1).
Positive contrast urethrography is difficult to perform in goats because of the urethral process. In the pig, it is also difficult to pass a urinary catheter in a retrograde fashion because of the spiral nature of the penis. For this reason, positive contrast, normograde urethrography via a tube cystotomy is recommended. This procedure entails placing a percutaneous cystotomy tube and approximately 50-200 milliliters of iodinated contrast medium (e.g. Hypaque-76) into the urinary bladder. The bladder is filled with sterile saline until the urine overflows into the urethra or the urinary bladder is palpable. A liter of saline may be required before urination occurs and the urethra is opacified. Gentle pressure can be placed on the urinary bladder to promote emptying. This procedure provides a positive contrast cystogram and a positive contrast urethrogram.
Thoracic and abdominal radiography, especially in large food animals, requires specialized equipment generally found only in teaching or referral hospitals. The reticulum can be imaged with a portable unit if the cow is first placed in dorsal recumbency. This moves the reticulo and abomasal contents toward the back of the animal, and the air rises to outline the ventral position of the reticulum (see Figure 10.3-4). Alternate imaging modalities, such as ultrasonography, should be considered for evaluating the thorax and abdomen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree