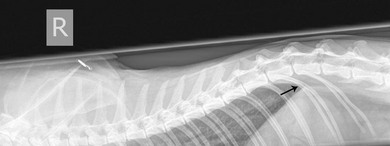
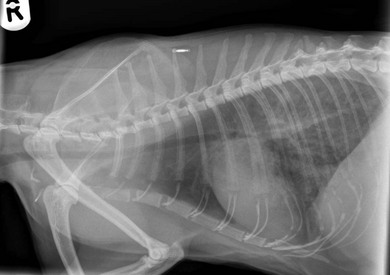
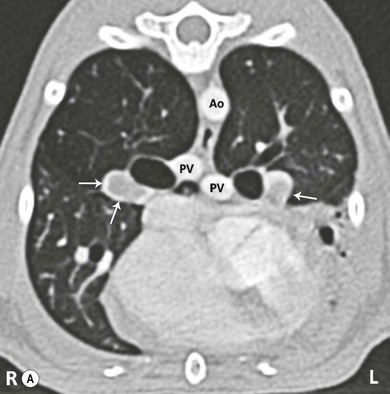
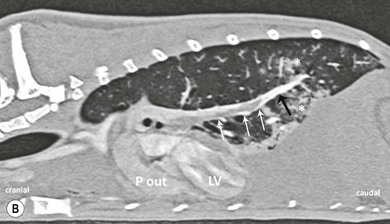
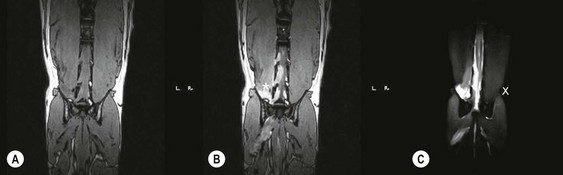
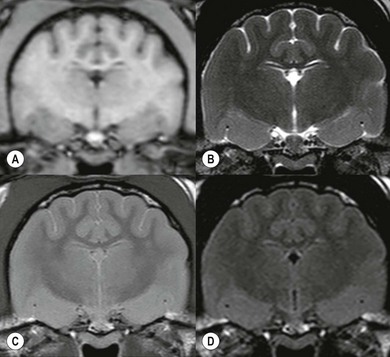
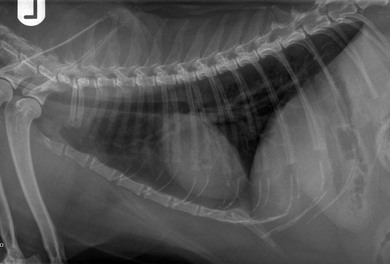
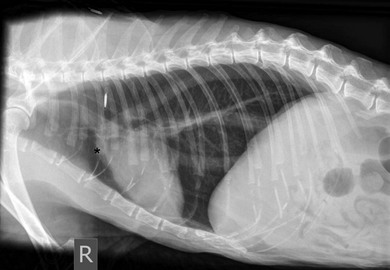
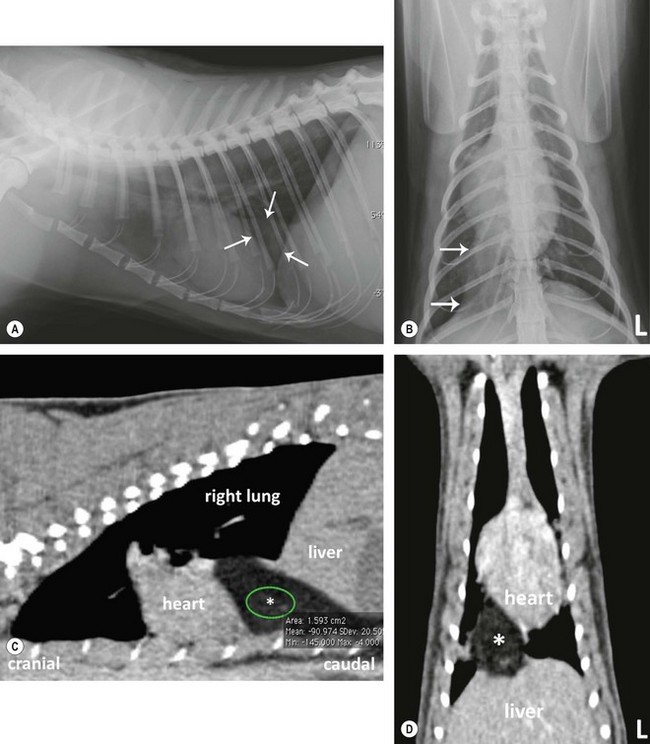
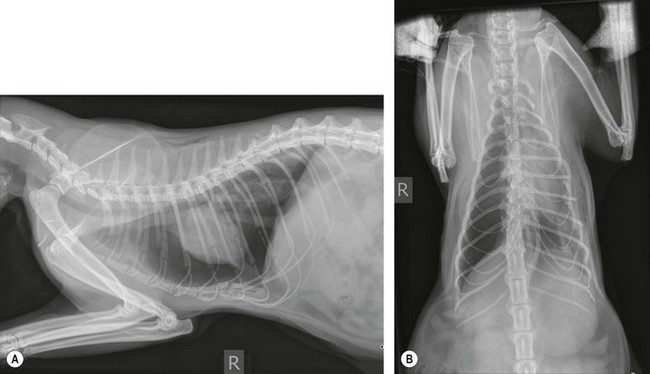
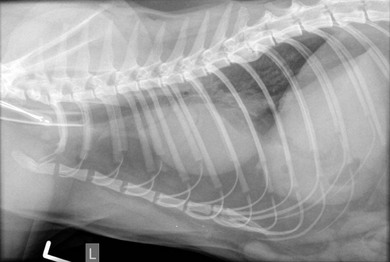
Chapter 8 Diagnostic imaging forms an essential part of the investigation for many general and oncological diseases in cats. Radiology and ultrasound remains the mainstay for initial investigations for many conditions, but with the advent of easier accessibility to advanced imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) these modalities are becoming used more frequently. The indications for each imaging modality for investigation of thoracic and abdominal disease and for imaging the brain will be covered in this section. For further general information on diagnostic imaging and specifically radiography of bone diseases, the reader is referred to other specialized texts1 and the imaging chapter in Feline Orthopedic Surgery and Musculoskeletal Disease.2 Due to an increasing availability and the advantages of multi-slice CT (fast acquisition with large anatomic coverage, thin slice thickness, increased spatial resolution), this technology has also become a highly valuable tool in veterinary medicine. As a cross-sectional imaging technique, CT avoids superimposition of adjacent structures by imaging a transverse slice. Consequently, soft tissue structures enveloped by bones e.g. the nasal cavity, brain, or pelvic organs, may be imaged well with CT whereas a radiographic image of these regions is of limited value. CT has also been increasingly used for imaging the lung, mediastinum and various abdominal organs. CT angiography is easy to perform with a peripheral intravenous injection of contrast medium (Fig. 8-1). With multi-slice CT, an arterial and venous phase, and for the liver a portal phase, can be imaged. In oncological diseases, CT angiography may indirectly help to identify and describe a neoplastic lesion on the basis of its relationship to adjacent arteries or veins (mass effect, compression and invasiveness), or the lesion itself may be outlined on the basis of its individual perfusion/contrast enhancement pattern. Figure 8-1 CT-angiography. (A) transverse plane and (B) left sagittal plane of a 9-year-old cat with immune-mediated hemolytic anemia and sudden onset of dyspnea and right heart failure. The pulmonary veins (PV) and the aorta (Ao) show homogeneous contrast filling; a large filling defect is seen in the right and left caudal pulmonary arteries (white arrows) consistent with thrombosis. Caudal to the thrombus, the arteries are again filled with contrast medium (black arrow). Secondary lung changes also occurred (*). P out, pulmonary outflow tract; LV, left ventricle. MRI has long been the imaging modality of choice for neurologists and it is now also becoming a more important imaging modality for other clinical specialties. MRI has great potential for increasing use in other disease complexes, especially if soft tissue components need to be assessed, quantified and qualified. MR imaging has the potential to have a great impact in oncological and endocrinological disease in feline patients although relatively few studies exist in cats to date (Fig. 8-2). Figure 8-2 Paraspinal lymphoma invading into the spinal canal in a cat. Dorsal T1-weighted images (A) pre- and (B) post-administration of gadodiamide (gadolinium) and (C) dorsal STIR. In the T1-weighted image the space-occupying lesion is barely visible, whereas in the contrast enhanced image the extent of the lesion is much clearer (asterisk). Note the absence of fat signal in the STIR (x). MRI generates images without the use of X-rays. Instead it uses the magnetic properties of protons distributed in the patient’s body. Two MRI systems are currently in standard use in veterinary medicine, namely low-field systems (low magnetic field strength) using open scanners, and closed-bore systems with stronger magnetic fields. The open systems offer better access to the patient, the higher magnetic fields give better local resolution with slightly shorter scanning times. The higher the magnetic field, the more artefacts are possible. The high-field scanners (>1 Tesla) usually provide a large field of view (up to 50 cm) giving the possibility of performing whole-body imaging, which is excellent for tumor staging. Sequences used for staging include the fast turbo short tau inversion recovery (STIR) imaging,3 which is widely used in human medicine nowadays. Different sequences can be used during MRI. The recorded signal is analyzed by a computer using a complicated calculation method and eventually presented to the radiologist in the form of a section image.4 Depending on the form, duration and intensity of the high-frequency impulses emitted into the region of interest (‘pulse sequence’), the tissue contrast in the image can be determined primarily through different T1- or T2-relaxation times, but also through the proton density alone. In this context, we refer to T1-, T2- or proton density (PD)-weighted sequences. Depending on these weightings, a tissue may be visualized with different signal intensities. For example, pure water shows low signal intensity in T1-weighted sequences, and high signal intensity in T2-weighted sequences. In contrast, fat shows up with high signal intensity in both weightings, whereas muscle shows medium signal intensity in T1-weighted images, but low signal intensity in the T2-weighted ones (Fig. 8-3). The tissue contrast can be manipulated even further using special sequences. An example is the STIR sequence allowing selective suppression of the fat signal with high signal visualization of fluids in basically a T2-weighted sequence. The same can be done to extract the signal of cerebrospinal fluid (CSF) in T2-weighted images of the central nervous system. This is done with FLAIR-sequences (fluid attenuation inversion recovery) (Fig. 8-3). Figure 8-3 Comparison of (A) T1, (B) T2, (C) PD and (D) fluid attenuated inversion recovery (FLAIR) of the feline brain. Note the absence of fluid signal in the T1 and the FLAIR images, whereas in the T2 image the fluid is bright in signal. In the fluid-sensitive sequences (T2, FLAIR), the gray matter shows lower signal intensity than the fluid, whereas it is the opposite in T1 images. The PD, which displays the density distribution of protons, shows perfect anatomical contrast. Like CT and ultrasound scanning, MRI is a tomographic (slice) method for producing a two-dimensional image. For CT the two-dimensional images are formed by reconstructing the sagittal and dorsal planes from the acquired transverse slices. MRI, in contrast, is a true three-dimensional modality, which can acquire each plane by itself without the need for post-scan reconstruction.5,6 The high tissue contrast in MRI is a major advantage compared with X-ray techniques (radiography, computed tomography). Also, tissues can be distinguished by the different intensities created by using different sequences, allowing far better assessment of soft tissue disorders (Table 8-1). MRI is a very sensitive modality, which still lacks specificity. However, the combination of different sequences, with and without contrast enhancement, provides a great tool for depiction and description of the extent of a lesion. This modality is useful for optimal surgery planning. Table 8-1 Representative tissue types with their signal behavior in T1 and T2 images Atelectasis commonly occurs in the dependent lung in lateral recumbency, which can mimic or mask lung pathologies. The DV view should therefore be taken prior to lateral views. Anesthesia-induced atelectasis can be minimized by placing the patient in sternal recumbency as soon as possible after induction.7–9 The extra-thoracic region includes the thoracic skeleton and the soft tissue of the thoracic wall and diaphragm. These structures should be included in a thorough evaluation of thoracic radiographs, in particular following trauma. Each individual rib, vertebral body and any of the included forelimbs should be scrutinized to identify skeletal lesions. Incompletely and densely mineralized costal cartilages might mimic fractures in older cats (Fig. 8-4). The thoracic surface of the diaphragm is visualized as it projects against the air-filled lung; caudally it silhouettes with the liver. In most cats, the ventral portion of the diaphragm is seen on a lateral view as a thin, soft tissue structure, outlined by mediastinal fat cranially and falciform fat caudally. Figure 8-4 Incompletely mineralized costal cartilages in older cats can be confused with rib fractures. The pleural space contains only a very small quantity of fluid that is not usually visible radiographically. Space-occupying abnormalities can distend the pleural space, e.g., fluid or air accumulation, pleural masses and herniated abdominal content. In cats, unilateral pleural diseases are more common than in the dog. In contrast to dogs, the caudodorsal lung fields are separated by a triangular-shaped soft tissue opacity from the spine on a lateral view. This represents the intrathoracic psoas muscles and should not be misinterpreted as pleural effusion (Fig. 8-5). Mediastinal structures normally seen radiographically include the heart, trachea, aorta, caudal vena cava, sometimes the esophagus, and in young animals the thymus. The normal feline heart is more consistent in shape than in dogs, with no breed-associated variations. The maximum width of the cardiac silhouette should not exceed 2–2.5 intercostal spaces on a lateral view. The apex of the heart is usually closer to the midline on a DV/VD view. In cats, the left atrium has a more cranial position, which makes it more difficult to identify on a lateral view. In older cats, the heart commonly becomes more horizontal in position and a focal aortic bulge might be noted (Fig. 8-6). In obese animals, large deposits of pericardial and mediastinal fat may mimic an enlarged cardiac silhouette. The cranial mediastinum should normally not exceed the width of the superimposed thoracic spine on a DV/VD view. Sternal and tracheobronchial lymph nodes are not visualized unless enlarged.7–12 Figure 8-6 Thoracic radiograph of a 14-year-old female cat. The heart is relatively horizontal in position. The descending aorta has a meandering course and a focal aortic bulge can be seen, indicated by an asterisk. The radiopaque linear metal density object is an overlying identity chip in the cat’s subcutaneous tissues. Computed tomography may be useful in cats with thoracic disease where other diagnostic modalities such as radiography and ultrasonography have failed to identify the cause or extent of the disease (Fig. 8-7). CT has potential for providing additional information on a variety of thoracic conditions in cats, particularly the possibility of identifying pulmonary metastases that are not visible on thoracic radiographs (Box 8-1). Figure 8-7 An opaque structure of fat to soft tissue density (arrows) was diagnosed radiographically in the cardiophrenic angle and on the right in a three-year-old cat with respiratory distress. The diaphragmatic contour was clearly seen in the (A) laterolateral view but not completely visualized on the (B) ventrodorsal view. It was unclear if the changes were consistent with lung pathology or a diaphragmatic hernia. With CT, a large defect was seen in the diaphragm on the right (C) dorsal plane and (D) ventrally (sagittal plane). The cardiophrenic angle was filled with fat (*, mean of −91 Hounsfield units). Findings were confirmed surgically. Echocardiography is the most sensitive imaging modality to diagnose and to classify cardiomyopathy. Echocardiographic changes are characterized by focal or generalized increased thickness of the left ventricular free wall and interventricular septum. Left atrial enlargement can usually be recognized in symptomatic patients. Thoracic radiographs, however, are indicated and superior to identify signs of congestive heart failure, such as pulmonary venous distension and pulmonary edema. In cats, pulmonary edema has a variable appearance and distribution (Fig. 8-8), rather than being located in the perihilar region as with dogs.13 Pleural effusion is often present secondary to left-sided heart failure. The concentric hypertrophy of the ventricular wall is often not recognized on radiographs and only an abnormal lung pattern might be noted. Advanced left atrial enlargement is particularly obvious on the DV view, seen as a ‘valentine’ shaped heart. Patients who have suffered thoracic trauma must be stabilized prior to radiography. In dyspneic patients a DV view should be taken to avoid further respiratory compromise. Lesions that might be recognized radiographically include pulmonary contusion, pneumothorax, and rib fractures (Fig. 8-9). Figure 8-9 Right lateral and ventrodorsal radiographs of a male neutered 5-year-old cat that presented with dyspnea after a road traffic accident. There is pneumothorax, with elevation of the heart, lung lobe consolidation and several rib fractures. Pulmonary contusion may be evident radiographically as complete lung lobe consolidation or as patchy areas of alveolar/interstitial infiltrates, commonly ipsilateral to the impact site. Radiographs taken immediately following trauma may not reveal these changes, which usually become apparent two-12 hours post-trauma.14 Diaphragmatic rupture is a common consequence of blunt external trauma. Identification of abdominal viscera in the thoracic cavity and loss of the diaphragmatic outline are conclusive signs for diaphragmatic rupture. However, pleural effusion may be present and obscure these structures. Cranial displacement and loss of abdominal organs on an abdominal radiograph are useful, indirect signs to identify a diaphragmatic rupture. In cats, only falciform fat and omentum may herniate, which may be recognized by the loss of the diaphragmatic line ventrally and by the relatively small fat pad ventral to the liver (Fig. 8-10).15 Positive-contrast gastrointestinal studies may aid the diagnosis. Figure 8-10 A 2-year-old female neutered obese cat presented with a pelvic fracture. Thoracic radiographs show loss of continuity of the diaphragm ventrally with elevation of the heart by a structure of fat density (suspected to be herniated falciform fat). There is also a soft tissue density structure lying ventral to the sternum, which was found to be a loop of intestine protruding through an abdominal wall rupture that was present in addition to the diaphragm rupture.
Diagnostic imaging
Diagnostic imaging modalities
Computed tomography
Magnetic resonance imaging
T1 image
T2 image
Tissue
Hypointense (dark)
Hyperintense (bright)
CSF, fluid collections, edema
Hyperintense
Hypointense
Fat, some protein solutions, calcium deposits, transient blood flow
Hyperintense
Hyperintense
Paramagnetic solutions, extracellular methemoglobin, proteins
Hypointense
Hypointense
Dense calcium deposits, bone, air, rapid flow in vessels, hemosiderin, metallic artefacts
Thorax
Radiology
Standard radiographic views
Interpretive principles and normal anatomy
Computed tomography
Specific thoracic conditions
Cardiomyopathy
Thoracic trauma
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diagnostic imaging