Harold C. McKenzie III,
Diagnostic Approach to Protein-Losing Enteropathies
Protein-losing enteropathy (PLE) is a clinical syndrome in which intestinal disease or dysfunction results in gastrointestinal tract protein loss and leads to hypoalbuminemia. Although this is often considered to be a discrete syndrome, many different types of intestinal disease can cause protein loss into the intestinal lumen; in many cases, however, the loss is too subtle to detect. Several pathophysiologic mechanisms can lead to enteric protein loss, including ulceration, inflammation, erosion, neoplasia, crypt cell proliferation, increased lymphatic pressure, and tight junction disruption. Malabsorption and maldigestion have the potential to reduce protein intake and absorption and may contribute to the development of hypoalbuminemia. It can be difficult to clinically differentiate PLE from malabsorptive disorders because both processes may be at work in an affected horse. Definitive diagnosis is rendered even more challenging in the equine patient because of the difficulty in gaining access to the intestine to obtain diagnostically useful intestinal biopsy specimens. Despite these challenges, the clinician must use the best information available to formulate a working diagnosis and develop an appropriate treatment plan.
Clinical Presentation
The most common clinical sign of PLE is dependent edema, which is primarily caused by the underlying hypoalbuminemia. The edema may be observed in some or all of the following areas: the mandibular region (bottle jaw), the ventral thorax and abdomen, or the distal limbs. The other signs associated with PLE are more variable but can include acute or chronic weight loss, diarrhea, and lethargy. Ascites, pleural effusion, or pericardial effusion may also be present in advanced cases. Clinical pathology will reveal the presence of hypoalbuminemia, which may be accompanied by low, normal, or high globulin concentrations. Total protein concentrations are often normal because of hyperglobulinemia or concurrent dehydration, but panhypoproteinemia may be present.
Differential Diagnoses
Enteric protein loss can be associated with a large number of conditions, including inflammatory bowel disease (IBD), infectious enteric disease, gastrointestinal ulceration, and endoparasitism. There is a constant turnover of proteins within the body, however, and the intestinal wall is not an impermeable barrier. It has been estimated that approximately 10% of the circulating albumin is lost into the intestinal lumen on a daily basis, but this loss is normally counterbalanced by hepatic production of albumin. Hypoalbuminemia develops when the rate of loss exceeds the rate of replacement, because of either increased enteric loss or decreases in the rate of hepatic production. As a result, many disease processes not involving the gastrointestinal tract can result in hypoalbuminemia or hypoproteinemia, including protein-losing nephropathies and hepatic insufficiency. External hemorrhage will also deplete systemic protein stores and may lead to panhypoproteinemia. Conditions involving third-space accumulation of fluid, such as peritonitis and pleuritis, may also result in decreases in serum albumin concentration. Severe protein or calorie malnutrition can lead to hypoproteinemia and hypoalbuminemia secondary to decreased protein production, as can malabsorptive and maldigestive disorders, which limit nutrient uptake.
Four basic types of pathophysiologic processes result in enteric protein loss: nonerosive gastrointestinal disorders, erosive gastrointestinal disorders, conditions involving mesenteric lymphatic obstruction, and disorders that increase central venous pressure. Most cases of PLE in the horse are associated with erosive or nonerosive conditions of the gastrointestinal tract. A multitude of syndromes fall under the heading of nonerosive disorders, with IBD being the most common, whereas infiltrative bowel diseases, endoparasitism, and proliferative enteropathy are less common causes of PLE. Infectious colitis or enterocolitis may also be associated with development of PLE, although these conditions are usually associated with a multitude of clinical challenges in addition to enteric protein loss. The erosive disorder most commonly associated with PLE is right dorsal colitis, but ulceration or erosion of the stomach, small intestine, or other segments of the large intestine may also be associated with PLE. Rare cases of mesenteric lymphatic obstruction may arise, most often resulting from mesenteric neoplasia. Increased central venous pressure can be caused by severe hepatic disease and congestive heart failure.
Diagnostic Approach
Many animals with PLE present with nonspecific clinical signs, which is a challenge for the clinician attempting to arrive at a diagnosis. Collection of a detailed history is critical because most horses have chronic disease, and environmental influences can be important in some causes of PLE. A thorough physical examination is indicated, including thorough thoracic and cardiac auscultation. The only characteristic clinical sign associated with many cases of PLE is dependent edema, which is typically a result of hypoalbuminemia. It is important to run a serum chemistry profile in horses with dependent edema to determine whether hypoalbuminemia is present. Total hypoproteinemia may not always be seen, however, because the globulin concentration is not always decreased in parallel with serum albumin, and indeed it may even be increased as a result of chronic inflammation or dehydration. No other abnormalities are consistently seen on the serum chemistry profile in horses with PLE, although total calcium concentration is typically low with hypoalbuminemia. A complete blood count should be performed, as well, to detect systemic inflammation, anemia, or abnormal leukocyte numbers. Serum amyloid A or fibrinogen concentrations may be high in horses with active local or systemic inflammation, but these are nonspecific markers.
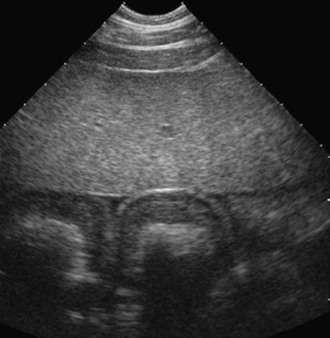
Abdominal ultrasonography is an important part of the evaluation of a horse with PLE because it allows evaluation of bowel wall thickness in both the small and large intestines. Bowel wall thickening is often seen with inflammatory and infiltrative bowel disorders and is a prominent feature of proliferative enteropathy (Figure 73-1). Care must be taken when interpreting this finding, however, because bowel wall thickening can occur as a secondary phenomenon in severely hypoalbuminemic horses, even when gastrointestinal pathology is not present, and in cases with impairment of mesenteric lymphatic drainage. In addition to bowel wall thickening, the clinician should evaluate the abdomen for peritoneal effusion, which may be a manifestation of abdominal inflammation (peritonitis) or may be secondary to hypoalbuminemia or an impairment of lymphatic drainage (ascites). The liver should be examined for evidence of fibrosis, congestion, or other abnormalities. The thorax should be evaluated, as well, primarily for pleural effusion, and if congestive heart failure is a possibility, a thorough echocardiographic exam should also be performed. Abdominal radiographs may be useful in cases in which intraabdominal masses or abscesses are suspected, or when foreign material may be present within the gastrointestinal lumen (e.g., sand enteropathy, gravel impaction).
Additional diagnostic testing may be useful, depending on initial examination findings, and can include abdominocentesis, gastroscopy, duodenoscopy, duodenal biopsy, rectal examination, and rectal biopsy. Abdominocentesis is indicated when increased peritoneal fluid volume or abnormal-appearing intestine is detected on abdominal ultrasound examination but is often performed as part of a thorough evaluation even when abnormalities are not detected on the ultrasound exam. Given the fact that the peritoneal fluid bathes all of the abdominal viscera, it is possible to detect inflammatory changes in the peritoneal fluid even when no other abnormalities are evident. High nucleated cell counts (>2500 cells/µL) are indicative of peritoneal inflammation, and the type of cells present may be indicative of underlying pathology. High protein concentrations (>2.5 g/dL) are indicative of peritoneal inflammation, whereas low protein concentrations may be an indication of ascites. The presence of abnormal cells in the peritoneal fluid may suggest abdominal neoplasia, although this is not highly sensitive because some abdominal neoplasms are not exfoliative. The presence of bacteria in the peritoneal fluid is indicative of peritonitis, particularly if large numbers of intracellular, bacteria are observed on cytologic examination. In severe or refractory cases of PLE, there may be an indication for abdominal exploratory surgery to include the collection of full-thickness intestinal biopsy specimens and appropriate mesenteric lymph node biopsy specimens.
Gastroscopy is useful for ruling out gastric ulcers, although gastric lesions are rarely responsible for enough protein loss to lead to clinically evident PLE. Gastric ulceration may be seen in horses with PLE, but it most likely represents a secondary development rather than being a primary etiology. The more important indication for gastrointestinal endoscopy in cases of PLE is duodenoscopic assessment of the small intestine for inflammation or ulceration. Duodenal abnormalities can be detected in some cases of PLE and may be associated with the primary disease syndrome. Inflammation, erosions, ulcers, scarring, and masses are all potential findings when examining the duodenal mucosa. Duodenoscopy also provides the opportunity for collection of duodenal mucosal biopsy specimens, which can be obtained with endoscopic biopsy forceps. It is recommended to collect two or three specimens, rather than a single sample, to increase the likelihood of obtaining a diagnostic sample. The primary limitation of endoscopic biopsy of the duodenum is that it can be difficult to obtain samples that penetrate deep into the mucosa, and the primary site of inflammation or infiltration often lies within the submucosal layers.
Rectal palpation is indicated in the evaluation of most horses with PLE when it is feasible and appropriate. Palpation may reveal intraabdominal masses, intestinal malpositioning, intestinal distension, or intestinal thickening. Transrectal ultrasonography may also be helpful, particularly if abnormalities are detected on the rectal examination. Transrectal ultrasonography often yields higher resolution images than transabdominal ultrasonography and will allow examination of areas of the caudal abdomen that cannot be imaged by use of the transabdominal approach. Rectal palpation also allows for rectal mucosal biopsy, which can reveal inflammatory or infiltrative processes. Rectal mucosal biopsy is easy to perform, and because the specimens derived are much larger and penetrate more deeply into the submucosa, it may be easier to detect pathology than with duodenal specimens. The limitation to rectal biopsy is that the sample is obtained from a site that is often remote from the primary site of disease in the small or large intestine. For that reason, changes may not be observed in the rectal tissue, or the changes observed may not be relevant to the primary disease process. Despite these limitations, rectal mucosal biopsy should be considered in the evaluation of all horses with PLE.
Additional diagnostic tests to consider include fecal flotation for parasite ova, fecal culture for Salmonella spp, fecal polymerase chain reaction (PCR) testing for Lawsonia intracellularis, fecal toxin testing for Clostridium difficile and Clostridium perfringens, and serology for Lawsonia spp. Feces can also be tested for occult blood and for albumin, although the true sensitivity and specificity for these tests in the horse are debated. Detection of blood suggests bleeding ulceration somewhere in the gastrointestinal tract, but is not specific for location. The presence of albumin may suggest erosive or ulcerative disease but may also indicate secretory losses from an active or passive process unrelated to loss of mucosal integrity. In humans and small animals, fecal testing for α1-proteinase inhibitor is used for the early detection of intraluminal protein loss, and it is sensitive enough to detect losses even before the development of systemic hypoalbuminemia. This test has not yet been validated for use in horses.
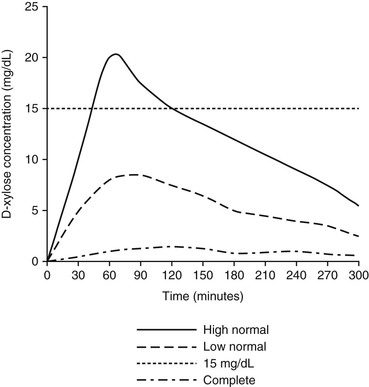
In addition to determining whether there are physical or structural abnormalities in the intestine, it is important that the clinician attempt to assess intestinal function. In many cases, intestinal absorption tests provide the most useful diagnostic information out of all the ancillary diagnostic tests that can be performed. The gold standard of absorptive tests is the D-xylose absorption test because this compound is not produced or metabolized by the body, rendering it unsusceptible to any metabolic influences. This test is performed by fasting the horse for 12 to 18 hours and administering 0.5 g/kg body weight of D-xylose, given as a 10% solution in water through a nasogastric tube. Blood samples are collected in heparinized tubes for D-xylose determination every 30 minutes, starting immediately before administration and out to at least 180 minutes. The D-xylose concentration should increase to a peak concentration of at least 15 mg/dL above baseline in horses with a normal GI tract, and this concentration should peak at 90 to 180 minutes (Figure 73-2). Flattening of the D-xylose concentration curve is indicative of malabsorption, whereas a delay in the peak suggests delayed gastric emptying.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree