CHAPTER 7 Diagnosis and Treatment of Feline Infectious Peritonitis
Feline infectious peritonitis (FIP) is an immune-mediated disease, triggered by infection with a feline coronavirus (FCoV). FIP is a major challenge to every veterinarian treating cats, because it is (1) very frequent, (2) sometimes very difficult to diagnose, (3) almost impossible to prevent, at least in multiple-cat environments, and (4) almost inevitably fatal within a short period of time. FIP is seen by primary veterinarians as often as by specialists. Approximately one of every 200 new feline cases presented to American veterinary teaching hospitals are cats with FIP.1 FIP is one of the most—if not the most—important reasons for death by infection in cats at this time.
FCoV belongs to the family Coronaviridae of the Order Nidovirales, a group of large, spherical, enveloped, positive-sense, single-stranded ribonucleic acid (RNA) viruses that are found very frequently in cats. With a genome of 27 to 32 kb, encoding for an approximately 750 kDa polyprotein, four structural proteins, and up to five accessory nonstructural proteins, coronaviruses are the largest RNA viruses known to date. FCoV can be subdivided into two types. Type I virus is the most prevalent in the field; type II virus is less common and results from recombination between type I FCoV and canine coronavirus involving the spike gene.2 Both types of viruses can induce FIP.
FCoV is transmitted fecal-orally between felids but is not infectious to other species (including human beings). Coronavirus-specific antibodies are present in up to 90 per cent of cats in catteries and in up to 50 per cent of cats in single cat households; however, only about 5 per cent of FCoV-infected cats will develop FIP in multiple-cat households.3
Initially it was hypothesized that FCoV strains causing FIP are different from avirulent enteric FCoV strains, and FCoV strains had been subdivided into two distinct “biotypes,” feline enteric coronavirus (FECV) and feline infectious peritonitis virus (FIPV). However, it is now known that all FCoV types may induce systemic infection as demonstrated by reverse-transcriptase polymerase chain reaction (RT-PCR) studies and that those “biotypes” are not two different virus species, but only represent virulence variants of the same virus.4
The precise process by which FIP develops is unclear, but there are two main hypotheses.5 The first hypothesis, the so-called internal mutation theory, is based on the fact that a mutation is necessary that favors viral replication in macrophages.6–9 In this case, cats are infected with the primarily avirulent FCoV, which replicates in enterocytes. In some instances, however, a mutation occurs in a certain region of the FCoV genome that confers to a new phenotype, leading to the ability of the mutated virus to replicate within macrophages. Although called internal mutation theory, no consistent mutation has been identified to date. In support of this hypothesis is the presence of highly virulent strains of FCoV that are capable of inducing FIP consistently, albeit under experimental conditions.10 The second hypothesis for the development of FIP is that any FCoV can cause FIP but that viral load and the cat’s immune response determine whether FIP will develop.9–14 It is likely that both factors, namely viral genetics and host immunity, play a role in the development of FIP. In both hypotheses, the key pathogenic event in the development of FIP is the massive replication of FCoV in macrophages. If the cat fails to eliminate the macrophages infected with the replication-competent virus early in infection, the presence of the virus within macrophages initiates an ultimately fatal Arthus-type immune-mediated reaction that defines FIP.
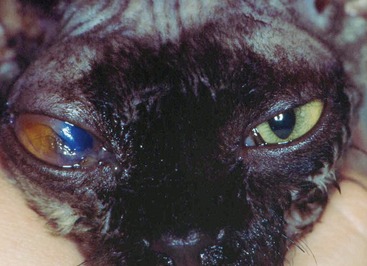
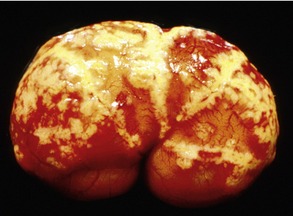
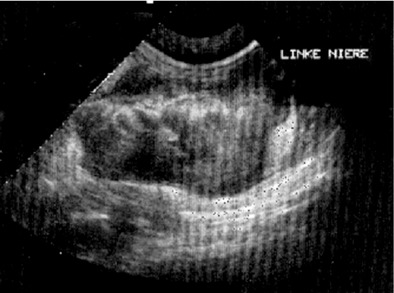
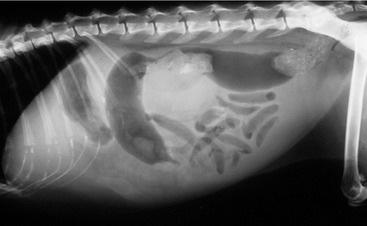
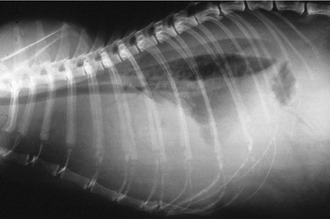
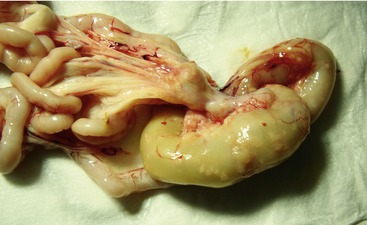
Affected cats will develop signs caused by (1) granulomatous lesions in target organs, including central nervous system (Figure 7-1), eyes (Figure 7-2), and parenchymatous organs (Figure 7-3), that usually are small and only in exceptional cases visible by diagnostic imaging methods (Figure 7-4); and (2) vasculitis leading to fluid redistribution into second spaces with fluid accumulation in body cavities manifesting as ascites (Figures 7-5 to 7-7) and thoracic (Figures 7-8 to 7-10) as well as other effusions (e.g., pericardial effusion, scrotal effusion). A rare nodular enteric form (Figure 7-11), seen in young cats with diarrhea and vomiting, is associated with intestinal granulomatous lesions.15 In addition to the well-known clinical presentations, some unusual pictures have been described. Skin fragility syndrome was found in a cat with FIP,16 and other skin lesions (e.g., nodular skin lesion, papular skin lesions, pododermatitis) may be present as well.17,18 One cat presented with priapism, and FCoV antigen was demonstrated immunohistochemically in its penile tissue.19
DIAGNOSIS
HEMATOLOGY AND SERUM BIOCHEMISTRY EVALUATION
Hematology results often are abnormal in cats with FIP, but the changes are not pathognomonic. White blood cell counts can be decreased or increased. Lymphopenia commonly is present, mainly caused by apoptosis of uninfected T cells, primarily CD8+ T cells, as a result of high tumor necrosis factor-α (TNFα) concentrations produced by virus-infected macrophages.20 However, lymphopenia in combination with neutrophilia generally is common in cats as a stress leukogram, is not typical for FIP, and can occur in many severe diseases in cats. A mild to moderate nonregenerative anemia also is a common finding in cats with FIP, but is another nonspecific laboratory abnormality that may occur in almost any chronic disease in cats.
The most common laboratory abnormality in cats with FIP is an increase in total serum protein concentration caused by increased globulins, mainly γ-globulins.21,22 In one study, hyperproteinemia was present in about 50 per cent of cats with effusion and in 70 per cent of cats without effusion.23 After experimental induction of FIP, an early increase of α2-globulins is present, while γ-globulins and antibody titers increase only just before appearance of clinical signs.24,25 The characteristically high levels of γ-globulins and the increased antibody titers invite the conclusion that hypergammaglobulinemia is due to a specific anti-FCoV immune response. Antibody titers and hypergammaglobulinemia show a linear correlation, but the wide variation in anti-FCoV titers at a given concentration of γ-globulins indicates that additional (autoimmune) reactions play a major role in the pathogenesis of FIP. It has been discussed that stimulation of B cells by interleukin-6, which is produced as part of the disease process, contributes to the increase in γ-globulins.26 Thus, total protein in cats with FIP can reach very high concentrations of up to 120 g/L (12 g/dL) and higher. Albumin-to-globulin ratio, however, has a significantly higher diagnostic value to differentiate FIP from other diseases than total serum protein or γ-globulin concentrations, because a decrease in serum albumin also may occur through a decrease in production in association with liver failure or protein loss.27,28 Protein loss in cats with FIP is caused by glomerulopathy secondary to immune complex deposition, by loss of protein due to exudative enteropathy in the case of granulomatous changes in the intestines, or by extravasation of protein-rich fluid during vasculitis.28 An optimal cut-off value of 0.8 was determined for the albumin-to-globulin ratio.28
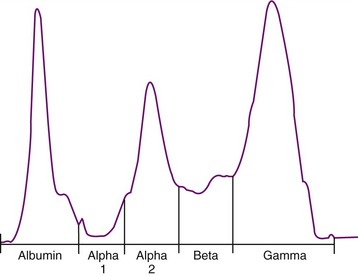
Serum protein electrophoresis also is often performed in cats suspected to have FIP (Figure 7-12); the main rationale behind the test is to distinguish a polyclonal from a monoclonal hypergammaglobulinemia in order to differentiate FIP (and other chronic infections) from tumors such as multiple myelomas or other plasma cell tumors (see Chapter 65). However, in cats with FIP, both polyclonal and monoclonal hypergammaglobulinemia can occur, and the same is found in tumors. Therefore the value of electrophoresis is limited.
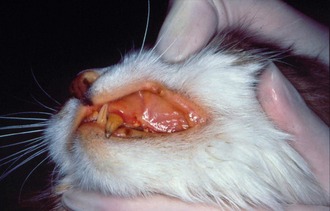
Other laboratory parameters, including liver enzymes, bilirubin, blood urea nitrogen, and creatinine, can be elevated variably depending on the degree and localization of organ damage but are not helpful in establishing an etiological diagnosis. Hyperbilirubinemia and icterus (Figure 7-13) often are observed and frequently are a reflection of hepatic necrosis. Sometimes bilirubin concentration is increased in cats with FIP without evidence of hemolysis, liver disease, or cholestasis; this unusual finding is otherwise observed only in septic animals. Bilirubin metabolism and excretion into the biliary system is compromised in these cats, likely due to high levels of TNFα that inhibit transmembrane transport. Thus, high bilirubin in the absence of hemolysis and elevation of liver enzyme activity should raise the suspicion of FIP.28
Recent research has focused on the diagnostic value of acute-phase reaction parameters including α1-acid glycoprotein (AGP), a serum acute phase protein that is elevated in cats with FIP.29,30 AGP is not only overexpressed in cats with FIP but also undergoes several modifications in the sialic acid content, including decreased expression of both α(2,6)-linked and α(2,3)-linked sialic acid.31 High serum AGP levels (>3 mg/mL) can support the diagnosis of FIP,32 but levels also rise in other inflammatory conditions, and thus these changes are not specific. Additionally, AGP also may be high in asymptomatic cats infected with FCoV, especially in households in which FCoV is endemic.33
EFFUSION FLUID EXAMINATION
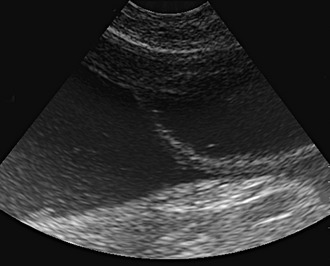
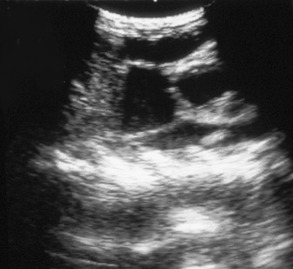
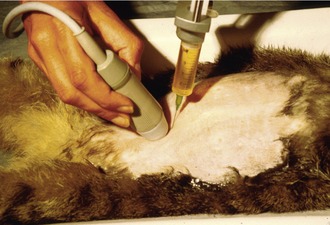
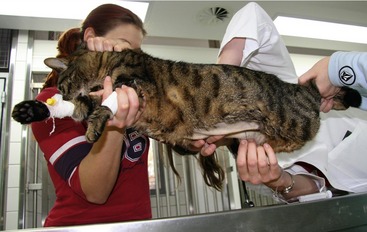
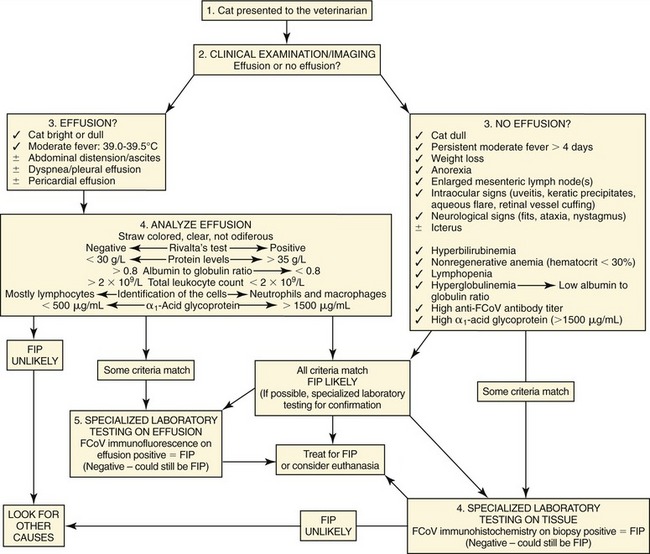
If there is effusion, the most important diagnostic step is to get a fluid sample because tests on effusion have a much higher diagnostic value than tests that can be performed on blood. Fluid can be obtained through ultrasound-guided fine-needle aspiration (Figure 7-14) or using the “flying cat technique” in case of ascites (Figure 7-15). Only about one half of all cats who are presented with effusion have FIP.34 Thus, although effusions of clear yellow color (Figure 7-16) and sticky consistency often are called “typical,” the presence of this type of fluid in body cavities alone is not diagnostic. Sometimes the fluid caused by FIP has a totally different appearance, and even some cases with pure chylous effusion have been reported.35 Usually, the effusion’s protein content is very high (>3.5 g/dL) and consistent with an exudate, whereas the cellular content is low (<5000 nucleated cells/mL) and resembles more that of a modified transudate or even pure transudate.
Major differential diagnoses for these effusions include inflammatory liver disease, lymphoma, heart failure, and bacterial peritonitis or pleuritis. Lactate dehydrogenase (LDH) activity typically is high (>300 IU/L) in effusions due to FIP as it is released from inflammatory cells. Cytological examination of the effusion in cats with FIP shows a variable picture, but often consists predominantly of macrophages and neutrophils (Figure 7-17). Cytological changes may appear similar in cats with bacterial serositis or sometimes with lymphoma; these effusions, however, usually can be differentiated by the presence of malignant cells or intracellular bacteria in cytological specimens and by bacterial growth on culture, respectively. Electrophoresis on effusion fluid is a diagnostic tool with a relatively high positive predictive value (if albumin-globulin ratio is <0.4) and a relatively high negative predictive value (if the ratio is >0.8).
“Rivalta’s test” is a very simple, inexpensive method that does not require special laboratory equipment and can be performed easily in private practice. This test was developed originally by an Italian researcher (named “Rivalta”) around 1900 and was used to differentiate transudates and exudates in human patients. However, other methods have replaced this test in human medicine. It is not diagnostically helpful in dogs with effusion. However, this test is very useful in cats to differentiate between effusions due to FIP and effusions caused by other diseases.28 The high protein content and high concentrations of fibrin and inflammatory mediators lead to a positive reaction (Box 7-1). To perform this test (Figure 7-18), a transparent reagent tube (volume 10 mL) is filled with approximately 8 mL distilled water, to which one drop of acetic acid (highly concentrated vinegar, 98 per cent) is added and mixed thoroughly. On the surface of this solution, one drop of the effusion fluid is layered carefully. If the drop disappears and the solution remains clear, the Rivalta’s test is defined as negative. If the drop retains its shape, stays attached to the surface, or floats slowly down to the bottom of the tube (droplike or jellyfish-like), the Rivalta’s test is defined as positive (Figure 7-19). The Rivalta’s test had a high positive predictive value (86 per cent) and a very high negative predictive value for FIP (96 per cent) in a study in which cats who presented with effusion were investigated (prevalence of FIP 51 per cent).28 Positive Rivalta’s test results sometimes can occur in cats with bacterial peritonitis or lymphoma. Those effusions, however, usually are easy to differentiate through macroscopic and cytological examinations, and/or bacterial culture.
CEREBROSPINAL FLUID EXAMINATION
Analysis of cerebrospinal fluid (CSF) from cats with neurological signs due to FIP lesions may reveal elevated protein (50 to 350 mg/dL with a normal value of less than 25 mg/dL) and pleocytosis (100 to 10,000 nucleated cells/mL) containing mainly neutrophils, lymphocytes, and macrophages.36–38 This is, however, a relatively nonspecific finding. Many cats with neurological signs caused by FIP have normal CSF.
MEASUREMENT OF ANTIBODIES
Antibody titers measured in serum are an extensively used diagnostic tool. However, a high percentage of healthy cats are FCoV antibody–positive, and most of those cats will never develop FIP. There is no “FIP antibody test”; all that can be measured is antibodies against FCoV. Thus, antibody titers must be interpreted extremely cautiously; the inadequacies and pitfalls of this test have been the topic of continuous discussions and controversies, and it has been assumed that more cats have died of misleading FCoV antibody test results than of FIP.39 Although criticized frequently, antibody testing still has a certain role in the diagnosis and, more importantly, in the management of FIP, when done by appropriate methodologies and when results are interpreted properly. However, antibody testing can only be useful if the laboratory is reliable and consistent, because methodologies and antibody titer results may vary significantly. A single serum sample, divided and sent to five different laboratories in the United States, yielded five different results.40
It is important to understand that the presence of antibodies does not indicate FIP and the absence of antibodies does not exclude FIP. Low or medium titers have no diagnostic value.28 It has been shown that in cats with fulminant FIP, titers decrease terminally,39 and approximately 10 per cent of cats with clinically manifest FIP have negative results.28 This is either because large amounts of virus in the cat’s body bind to antibodies and render them unavailable to bind the antigen in the antibody test, or because the antibodies are lost into the effusion when protein is translocated during vasculitis. If interpreted carefully, very high titers can be of certain diagnostic value.
It also has been shown that the height of the antibody titer correlates directly with the amount of virus that is shed in the feces: cats with high antibody titers are more likely to shed FCoV and to shed more consistently higher amounts of the virus.41 Therefore the height of the titer is correlated directly with virus replication rate and the amount of virus in the intestines.
Measuring antibodies in fluids (e.g., effusion, CSF) other than blood has been investigated. Presence of antibodies in effusion is correlated with the presence of antibodies in blood,42 and thus, antibody titers in effusions also are not very helpful. Cats in a recent study had medium antibody titers irrespective of whether they had FIP or not.43 One study looking into the diagnostic value of antibody detection in CSF found a very good correlation to the presence of FIP when compared to histopathological examination44; however, two recent studies of a large number of cats presented to veterinary teaching hospitals revealed no significant difference in antibody titers in CSF from cats with neurological signs due to FIP compared to cats with other neurological diseases confirmed by histopathological examination.45,46
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree