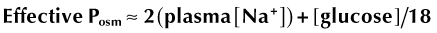CHAPTER 28 Diabetic Emergencies
Traditionally DKA and HHS have been described as two extremes of diabetic complications, with DKA characterized by ketosis and acidosis and HHS characterized by severe hyperglycemia and dehydration.1 In human beings DKA was believed to develop acutely, over a 24-hour period, in young patients with type 1 diabetes, whereas the HHS syndrome was thought to develop over a period of days to weeks in older patients with type 2 diabetes.1 However, more recently, it has become apparent that about 40 per cent of human patients with DKA also have hyperosmolarity and that many individuals develop a diabetic complication that is a mixture of DKA and HHS.1
DEFINITION AND PATHOPHYSIOLOGY OF DIABETIC KETOACIDOSIS
In nondiabetics acetyl-CoA and pyruvate enter the citric acid cycle to form ATP. However, in diabetics, glucose does not enter cells in adequate amounts and production of pyruvate by glycolysis is decreased. The activity of the citric acid cycle therefore is diminished, resulting in decreased utilization of acetyl-CoA. The net effect of increased lipolysis and acetyl-CoA production along with decreased utilization of acetyl-CoA in the citric acid cycle is an increase in the concentration of acetyl-CoA.2
The three ketone bodies synthesized from acetyl-CoA include β-hydroxybutyrate, acetoacetate, and acetone. Acetyl-CoA is converted to acetoacetate by two metabolic pathways, and acetoacetate then is metabolized to β-hydroxybutyrate or acetone. One of the pathways of acetoacetate synthesis involves condensation of two acetyl-CoA units and the other utilizes three units of acetyl-CoA. Ketone bodies are synthesized in the liver.2
Acetoacetate and β-hydroxybutyrate are anions of moderately strong acids. Therefore their accumulation results in ketotic acidosis. Metabolic acidosis and the electrolyte abnormalities that ensue are important determinants in the outcome of patients with DKA.3
One of the beliefs regarding the pathophysiology of DKA had been that patients who develop DKA have no or undetectable endogenous insulin concentration. While endogenous insulin concentration in cats with spontaneous DKA has not been reported, in one study five of seven dogs with DKA had detectable endogenous serum insulin concentrations, and two of these dogs had endogenous serum insulin concentrations within the normal range.4 Similarly, a recent study described a group of seven cats with DKA who went on to develop diabetic remission lasting from 5 weeks to 24 months.5 The ability to attain remission suggests that some cats with DKA have endogenous insulin secretory ability, allowing them to become independent of exogenous insulin therapy for various time periods. Therefore it is unlikely that zero or undetectable endogenous insulin concentration is a single important factor in the pathophysiology of DKA in cats.
A different factor that may be important in the pathophysiology of DKA is the presence of an elevated serum glucagon concentration, which could occur secondary to concurrent disease. While serum glucagon concentration in cats with spontaneous DKA has not been reported, a recent report of serum glucagon concentration in diabetic dogs found that a rise in glucagon concentration was significantly associated with an increase in ketone concentration.6
DEFINITION AND PATHOPHYSIOLOGY OF THE HYPEROSMOLAR HYPERGLYCEMIC STATE
HHS is defined as a severe diabetic metabolic complication and clinical decompensation characterized by profound hyperglycemia (≥600 mg/dL), hyperosmolarity (effective serum osmolality ≥320 mOsm/kg in cats or ≥330 mOsm/kg in human beings), and dehydration.1,7 HHS also is defined by the absence of ketoacidosis.1
The pathophysiology of HHS is incompletely understood, but is believed to be similar to that of DKA in that increased glucagon concentration plays an important role in its development. Similar to DKA, insulin concentration is not zero in HHS.1 It is not known why some diabetic individuals develop DKA whereas others develop HHS. The notion that HHS develops solely in older diabetic patients with type 2 diabetes mellitus has been refuted, because HHS has been reported in pediatric patients.1,8 As in human beings, severely compromised diabetic cats exhibiting a mixture of the classic characteristics of DKA and HHS have been described.8
RISK FACTORS FOR DKA OR HHS
The mean age of cats with DKA is 9 years (range, 2 to 16 years),9 whereas the mean age of cats with HHS is 12.6 ± 3.2 years.7 Specific breed or gender has not been shown to increase the risk of DKA or HHS in cats.7,9,10
Concurrent disease is common in cats with DKA or HHS. It is possible that the presence of concurrent disease results in elevated serum glucagon concentrations, which increase the risk of DKA or HHS. Concurrent disease has been documented in about 90 per cent of cats with DKA. The most common concurrent diseases noted in cats with DKA are hepatic lipidosis, chronic kidney disease, acute pancreatitis, bacterial or viral infections, and neoplasia. 9 In a study that compared cats with HHS to those with DKA or uncomplicated diabetes, about 90 per cent of cats with HHS had a concurrent disorder.7 Distribution of concurrent disease varied between disease states. Specifically, cats with HHS were significantly more likely to have chronic kidney disease or congestive heart failure as compared with cats with DKA or uncomplicated diabetes, and to have neoplasia or infections as compared with cats with uncomplicated diabetes (but not DKA).7 Infections noted in the group of cats with HHS were upper respiratory or urinary tract infections, otitis, a necrotic toe, severe purulent dental lesions, and gastrointestinal parasites.7 Acute pancreatitis was significantly more common in cats with DKA compared with cats with HHS or uncomplicated diabetes, and there was no significant difference in the rate of acute pancreatitis between cats with HHS and uncomplicated diabetes.7 The rate of corticosteroid administration was not significantly different among the three groups of cats.7
Most cats with DKA are newly diagnosed diabetics.9 However, about 70 per cent of cats with HHS were treated previously with insulin.7 There is no apparent significant difference between duration of diabetes prior to diagnosis of HHS or DKA.7,9
The two most common risk factors for development of DKA or HHS in human beings are inadequate or inappropriate insulin therapy and infection.1 Similarly 65 per cent of dogs with DKA are newly diagnosed diabetics who have not been treated previously with insulin, and 20 per cent have a urinary tract infection.
CLINICAL SIGNS AND PHYSICAL EXAMINATION FINDINGS IN CATS WITH DKA OR HHS
Clinical signs and physical examination findings may be attributed to chronic untreated diabetes, presence of concurrent disease, and the acute onset of DKA or HHS. The most common clinical signs of cats with DKA or HHS are polyuria and polydipsia, lethargy, inappetence or anorexia, vomiting, and weight loss.7,9 Additional clinical signs reported in cats with HHS include ataxia or weakness, respiratory problems, inappropriate elimination, or neurological signs (e.g., circling, pacing, and unresponsiveness).7
Common abnormalities noted on physical examination of cats with DKA are a subjectively underweight body condition, dehydration, icterus, or hepatomegaly.9 Common abnormalities noted on physical examination of cats with HHS are a subjectively overweight body condition, dehydration (noted in about 80 per cent of cats and classified as severe in about 50 per cent of cats with HHS), dental disease (noted in about 60 per cent of affected cats), respiratory compromise, and lower body temperature compared with cats with uncomplicated diabetes mellitus.
CLINICAL PATHOLOGY OF DKA OR HHS
Anemia and neutrophilia with a left shift are common features of feline DKA.9 Cats with DKA also have significantly more red blood cell Heinz body formation compared with normal cats, and the degree of Heinz body formation is correlated with plasma β-hydroxybutyrate concentration.11 In contrast, anemia is uncommon in cats with HHS and was observed in only three of 17 cats studied.7 Four of 17 cats with HHS had Heinz body formation, and mature neutrophilia was noted in about 50 per cent of cats with HHS. 7
Persistent hyperglycemia is apparent in all cats diagnosed with DKA or HHS, unless they have been treated with insulin.7,9 As the definition of HHS implies, cats with HHS have a blood glucose concentration of 600 mg/dL or greater. However, it is possible for a cat to have a blood glucose concentration of 600 mg/dL or greater with diabetes mellitus that is not complicated by HHS or DKA. Elevations in alanine aminotransferase (ALT) activity and cholesterol concentration have been reported in about 80 per cent of cats with DKA, but they are not usually elevated in cats with HHS.7,9,10 In comparison, aspartate aminotransferase (AST) activity was elevated in 11 of 11 cats with HHS.7 Azotemia has been reported in approximately 50 per cent of cats with DKA and in about 90 per cent of cats with HHS.7,9 Alkaline phosphatase (ALP) activity is normal in most cats with DKA or HHS, although it is elevated in almost all dogs with DKA.2,7,9
The pathophysiology of electrolyte abnormalities in cats with DKA and HHS differs between the two complications.1,7,9 In DKA whole body potassium depletion usually is present, but may not be apparent at presentation. Cats with DKA often have a decreased potassium intake as a result of inappetence or anorexia, and increased loss through vomiting and osmotic diuresis. Hypokalemia may be exacerbated by binding of potassium to ketoacids. On the other hand, serum potassium concentration may be increased as excess hydrogen ions shift from the extracellular fluid into cells. Positively charged potassium ions then are shifted out of cells to compensate for the electric change associated with movement of positively-charged hydrogen ions into cells. Hyperglycemia and hypoinsulinemia also contribute to a shift of potassium to the extracellular fluid. Initially a cat with DKA may even appear to have hyperkalemia because of decreased renal excretion, dehydration, and decreased insulin function. However, with rehydration, potassium ions are lost from the extracellular fluid and hypokalemia rapidly becomes apparent. Insulin therapy may worsen hypokalemia because insulin shifts potassium into cells. The most important clinical significance of hypokalemia in DKA is profound muscle weakness, which may result in ventroflexion of the neck and, in extreme cases, respiratory paralysis (see Chapter 59).
A study comparing serum potassium concentration of cats with DKA and HHS at the time of admission found that 36 cats with DKA had significantly lower potassium concentration (mean 3.1 ± 0.7 mmol/L) compared with 16 cats with HHS (mean 4.2 ± 0.9 mmol/L). In contrast, in human beings total body potassium depletion in HHS is thought to be more profound than in DKA.1 However, severe dehydration in patients with HHS may mask total body potassium depletion at the time of initial examination more than in patients with DKA.1 In 127 dogs with DKA, only 45 per cent of the patients were hypokalemic at the time of initial examination, but 84 per cent of dogs developed hypokalemia over the course of hospitalization.3 Future studies comparing potassium concentration in rehydrated cats with DKA and HHS are needed in order to improve our understanding of potassium depletion in these patients.
In DKA hypophosphatemia develops when phosphate shifts from the intracellular to the extracellular space as a result of hyperglycemia, acidosis, and hypoinsulinemia. Osmotic diuresis or fluid therapy along with insulin therapy cause extracellular phosphate depletion leading to whole body phosphate depletion. In HHS the most important factor in the pathophysiology of hypophosphatemia is severe osmotic diuresis. Hypophosphatemia related to DKA has been associated with hemolysis in a cat and with seizures in a dog.12 Additional clinical signs that may develop by reason of hypophosphatemia include weakness, myocardial depression, and arrhythmias. In human beings total body phosphate depletion in DKA is thought to be more profound than in HHS.1
Interestingly, while hypophosphatemia is recognized commonly in human patients with DKA and HHS and in dogs with DKA, hyperphosphatemia (rather than hypophosphatemia) actually is reported more commonly in cats with DKA and HHS.1,3,7,9 The reason may be that studies in cats report serum phosphate concentration measured only at the time of admission when dehydration may mask hypophosphatemia. Furthermore, many cats with DKA and HHS have chronic kidney disease, which may contribute to increased phosphate concentration.
Decreased plasma ionized magnesium (iMg) concentration has been documented in four of five cats with DKA, and may be caused by increased urinary magnesium excretion.13 The clinical significance of hypomagnesemia in cats is unknown, but in human diabetics it includes insulin resistance, hypertension, hyperlipidemia, and increased platelet aggregation. Dogs with DKA usually do not have low iMg concentration at the time of initial examination.3
Serum sodium concentration can be high, normal, or low in patients with DKA or HHS. In human beings profound osmotic diuresis usually leads to a low serum sodium concentration at the time of initial examination. However, severe dehydration can mask sodium losses and may result in an initially normal or high serum sodium concentration. On the other hand, severe hyperglycemia can contribute to a pseudohyponatremia. Extracellular hyperglycemia results in fluid shifting from the intracellular to the extracellular space, causing dilution of the extracellular sodium (i.e., pseudohyponatremia). Various equations can be used to correct for pseudohyponatremia (e.g., the addition of 1.6 mEq to the measured serum sodium value for each 100 mg/dL glucose >100 mg/dL). For example, if the measured serum sodium concentration is 135 mEq/L and blood glucose concentration is 400 mg/dL, the corrected serum sodium concentration is 135 + (1.6 × 3) or 139.8 mEq/L.1 Additionally, hyperlipidemia may interfere with sodium measurement and may contribute to a falsely decreased serum sodium concentration.
In one study of cats with HHS or DKA the median corrected sodium concentration of cats with HHS (159 mmol/L) was significantly greater than that (151 mmol/L) of cats with DKA.7 In another study that compared cats with DKA to those with uncomplicated diabetes mellitus, 34 per cent of cats with DKA had hyponatremia in comparison to only 8 per cent with uncomplicated diabetes mellitus.10 A third study found that 80 per cent of 42 cats with diabetic ketosis or diabetic ketoacidosis were hyponatremic.9 In the two latter studies sodium concentration was not corrected for glucose. Hypochloremia also has been reported in human beings with DKA or HHS, and in cats and dogs with DKA.1,3,7,9,10
In patients with DKA and HHS, glucosuria usually is found on urinalysis, and proteinuria also may be apparent. In DKA, ketonuria may not be detected because the nitroprusside reagent in urine dipsticks reacts with acetoacetate but not with β-hydroxybutyrate, which is the dominant ketone body in DKA. Measurement of serum β-hydroxybutyrate concentration is more sensitive than measurement of urine ketones.14 Urinary tract infections develop in about 13 per cent of diabetic cats.15 The most common bacterial isolate is Escherichia coli.15 In diabetic cats a urinalysis is a useful screening tool for a urinary tract infection, because most cats with a urinary tract infection have either white blood cells or bacteria identified in the urine sediment.15 (See Chapter 48 in the fifth volume of this series for a discussion on bacterial urinary tract infections.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree