Fig. 5.1
Schematic view of the metabolism of l-glutamate (l-GLU) in small intestine absorptive epithelial cells. This schema is related to the involvement of l-glutamate in the production of other amino acids as well as conversion of l-glutamate into α-ketoglutarate (α-KG) for entry into the Krebs cycle. OAA is oxaloacetate, l-ASP is l-aspartate, PYR is pyruvate, l-ALA is l-alanine, l-ORN is l-ornithine, l-CITR is l-citrulline, CP is carbamoylphosphate, P5C is pyrroline-5-carboxylate, PRO is l-proline
A number of studies have shown that a large proportion of l-glutamate is metabolized during its transcellular journey through pig enterocytes. Indeed, in pigs, virtually all the enteral l-glutamate is metabolized by the gut during absorption (Reeds et al. 1996). In a 7-kg piglet, an increase in l-glutamate concentrations was observed in portal and arterial blood plasma when the basal milk formula, administered enterally at a rate of 510 μmol kg/h, was supplemented with a relatively high dose of monosodium glutamate, i.e., 1,250 μmol/kg/h (Janeczko et al. 2007). Similarly, in larger (60 kg) pigs, transient portal and arterial hyperglutamatemia was observed when the diet (i.e., 800 g meal containing 142 g casein) was supplemented with 10 g monosodium glutamate (Blachier et al. 1999), indicating that only very large doses of l-glutamate may exceed the intestinal capacity to catabolize this amino acid.
It has been demonstrated that dietary l-glutamate is the most important contributor among substrates for mucosal oxidative energy metabolism in pigs (Stoll et al. 1999). ATP production and utilization are very active in pig enterocytes. Indeed, although the gastrointestinal tract represents only approximately 5 % of body weight, it is responsible for around 20 % of whole-body oxygen consumption (Vaugelade et al. 1994). The pig intestinal epithelial cells have notably high energy demand (Madej et al. 2002; Wu and Knabe 1995) due to the rapid renewal of the epithelium within few days (Wiese et al. 2003), thus requiring intense anabolic metabolism. Sodium extrusion at the basolateral membranes through the activity of Na/K ATPase following nutrient absorption may also represent a major ATP-consuming process in enterocytes (Buttgereit and Brand 1995).
The metabolic steps involved in l-glutamate utilization in pig enterocytes involve firstly transamination with oxaloacetate to produce alpha-ketoglutarate and l-aspartate (Blachier et al. 2009) (Fig. 5.1). l-glutamate can also be transaminated in the presence of pyruvate to produce l-alanine and alpha-ketoglutarate. Transamination appears to be the principal route by which l-glutamate is converted to alpha-ketoglutarate in pig enterocytes, because these cells have little capacity for the conversion of l-glutamate into alpha-ketoglutarate (and ammonia) through glutamate dehydrogenase (Madej et al. 2002). l-glutamate and l-glutamine are similarly oxidized by pig enterocytes (Blachier et al. 1999). However, for l-glutamine, this amino acid has to firstly enter the mitochondria in order to be degraded to l-glutamate and ammonia by the phosphate-dependent glutaminase activity (Wu et al. 1994, 1995a; Fig. 5.2).
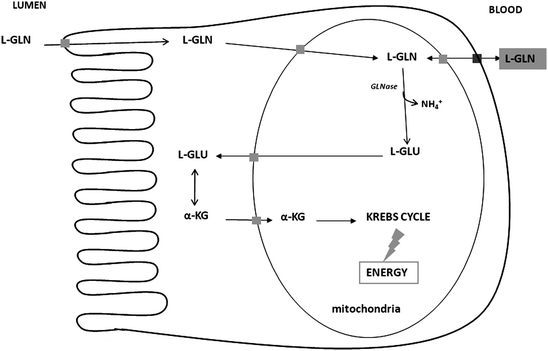

Fig. 5.2
Schematic view of the metabolism of l-glutamine (l-GLN) in small intestine absorptive epithelial cells. This schema is related to the metabolism of l-GLN to α-ketoglutarate (α-KG) for entry into the Krebs cycle. GLNase is glutaminase
l-glutamate that arises within the mitochondria can be either metabolized locally or exported into the cytosol, where the amino acid is metabolized into alpha-ketoglutarate by transamination before reentering the mitochondria and the tricarboxylic acid cycle (Duée et al. 1995). When both l-glutamate and l-glutamine are simultaneously presented to isolated pig enterocytes, l-glutamate can inhibit l-glutamine utilization and oxidation by inhibiting mitochondrial glutaminase activity (Blachier et al. 1999). Presumably, the sparing effect of l-glutamate over l-glutamine is dependent on the relative concentration of both amino acids in mitochondria.
l-glutamate and/or l-glutamine can be used by pig enterocytes to produce other amino acids including l-aspartate (Blachier et al. 1999), l-alanine (Wu et al. 1995a), l-proline (Wu and Knabe 1994), l-ornithine (Blachier et al. 1992), and l-citrulline (Wu et al. 1994) (Fig. 5.1). Note that neither ornithine nor citrulline is present in proteins. l-aspartate and l-alanine are thus produced in the course of glutamate catabolism in pig enterocytes. l-aspartate, either produced endogenously from glutamate/glutamine or originating from the exogenous luminal content, can enter mitochondria and is then oxidized in the TCA cycle (Windmueller and Spaeth 1976), thus representing another oxidative fuel for enterocytes. The stepwise conversion of l-glutamate to l-ornithine is performed in mitochondria. Then, l-ornithine can serve as a precursor for citrulline production (Blachier et al. 1991b). l-citrulline is then released into the portal vein and passes through the liver without major uptake and is then presumably used for de novo synthesis of l-arginine in kidneys (Flynn and Wu 1996).
Together with l-cysteine and glycine, l-glutamate is the precursor for the synthesis of glutathione in pig enterocytes (Fig. 5.3).
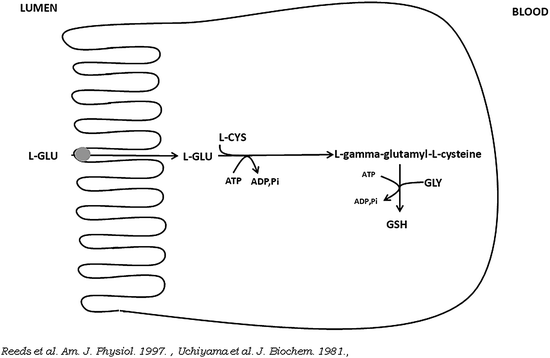

Fig. 5.3
Schematic view of the conversion of l-glutamate (l-GLU) into glutathione (GSH) in small intestine absorptive epithelial cells. l-CYS is l-cysteine, GLY is glycine
Studies in pigs suggest extensive utilization of dietary cysteine by the intestine (Stoll et al. 1998). It has been reported that in fed piglets, mucosal glutathione is derived largely from the direct metabolism of enteral l-glutamate (Reeds et al. 1997). The ratio of reduced to oxidized glutathione is an important measure for both the determination of the intracellular redox status and for the cell’s capacity to control intracellular concentrations of both oxygen-reactive and nitrogen-reactive species (Chakravarthi et al. 2006; Kemp et al. 2008).
Metabolism of l-glutamate and l-glutamine in enterocytes has been studied in growing piglets. Enterocytes isolated from piglet small intestine at birth display a high capacity for l-glutamine oxidation (Darcy-Vrillon et al. 1994), indicating that this amino acid can be used as a fuel at this stage of development when the growth of the intestine mucosa is very intense (Widdowson et al. 1976; Klein and McKenzie 1983a, b). Furthermore, enterocytes isolated from newborn pigs are able to convert l-glutamine into l-citrulline and then l-arginine (Blachier et al. 1993; Wu et al. 1994). This metabolic capacity of enterocytes may reflect the high content in sow’s milk proteins of glutamate and glutamine and the relatively low content of arginine (Wu and Knabe 1994). In suckling piglets, it has been observed that, soon after birth, the net enterocyte’s capacity to convert l-glutamine to l-citrulline is severely decreased compared with piglets at birth (Wu et al. 1995b). In contrast, l-glutamine still represents a major oxidative energy substrate for enterocytes in these animals. Indeed, in suckling piglet enterocytes, l-glutamine is approximately eight times more rapidly oxidized than in enterocytes isolated from weaned pigs (Darcy-Vrillon et al. 1994). In addition, conversion of enteral glutamate to proline in neonatal piglet gastrointestinal tract has been measured by Murphy (1996). Some interactions have been reported between glutamine and carbohydrate metabolism in piglet enterocytes. For instance, in these cells, the metabolism of d-glucose in the pentose cycle which allows NADPH synthesis appears to increase the net conversion of l-glutamine into l-proline (Wu 1996).
Colonic epithelial cells are also able to metabolize glutamate and glutamine. An important characteristic of the colonic epithelium, as said above, is that except for a short period after birth, there is little or no transfer of amino acids from the lumen to the portal blood (Darragh et al. 1994). Then, amino acids (including l-glutamate and l-glutamine) must be taken from arterial blood. Pig colonocytes can use l-glutamine as oxidative substrates (Darcy-Vrillon et al. 1994). Like in enterocytes, l-glutamine is firstly converted into l-glutamate and ammonia by the mitochondrial glutaminase, and then into alpha-ketoglutarate, mainly by transamination, followed by entry into the tricarboxylic cycle.
Colonocytes can also use luminal organic acids generated from the microbial activity, including short-chain fatty acids, as oxidative substrates (Darcy-Vrillon et al. 1993). Dietary substrates for short-chain fatty acid production are mainly dietary fibers, resistant starch, and some amino acids including l-glutamate (Bindelle et al. 2011; Htoo et al. 2007). Regarding amino acid metabolism by the microbiota, although alimentary protein digestion followed by amino acid and oligopeptide absorption by the small intestine is efficient in pigs (Stein et al. 1990), substantial amounts of nitrogenous compounds of both exogenous (alimentary) and endogenous origin may enter the pig large intestine through the ileocaecal junction. The first event in the large intestine protein degradation is hydrolysis of proteins and polypeptides by microbiota and residual pancreatic proteases and peptidases, which results in peptide and amino acid release, followed by the production of numerous bacterial metabolites. In the large intestinal lumen, l-glutamate released from proteins and peptides is the precursor for acetate and butyrate production (Blachier et al. 2007), but the relative contributions of l-glutamate and alimentary indigestible polysaccharide to acetate and butyrate production have not been determined. Glutamine synthetase, in terms of protein expression and catalytic activity, has been measured in pig colonocytes (Eklou-Lawson et al. 2009). Because the l-glutamine-degrading enzyme glutaminase is also highly expressed in colonocytes, this raises the open question of the physiologic meaning of the expression within the same cells of l-glutamine-synthesizing and -degrading enzymes. Because ammonia at concentrations that can be found in the pig colonic lumen inhibits short-chain fatty acid oxidation in colonocytes (Darcy-Vrillon et al. 1996), it has been proposed that cytosolic glutamine synthetase activity, which converts l-glutamate and ammonia into l-glutamine, may represent a way to reduce the intracellular concentration of ammonia during its transfer from the lumen to the bloodstream (Eklou-Lawson et al. 2009), thus avoiding metabolic disturbances.
Utilization of l-glutamate in colonocytes is not restricted to energy metabolism. Indeed, l-glutamine metabolism in pig isolated colonocytes leads to a net production of l-aspartate, l-alanine, and lactate (Darcy-Vrillon et al. 1993). However, unlike what is observed in enterocytes, pig colonocytes produce more l-aspartate than l-alanine from l-glutamine.
5.3 Metabolic Fate of Arginine (and Related Amino Acids, i.e., Ornithine, Citrulline, and Proline) in Pig Enterocytes and Colonocytes
In pig enterocytes isolated from postweaning animals, arginine is degraded mainly into urea, ornithine, and citrulline and to a much lesser extent to nitric oxide (NO) (Wu et al. 1995a; Blachier et al. 1993; M’Rabet-Touil et al. 1993, 1995). The synthesis of ornithine and urea from arginine is made from the catalytic activity of arginase. Although urea represents a metabolic end-product, a part of ornithine derived from arginine can be used as a substrate together with carbamoylphosphate to allow citrulline synthesis through the activity of carbamoylphosphate synthetase isoform I (CPS I). Citrulline can also be produced together with NO through the activity of nitric oxide synthase (NOS) which catalyzes the conversion of arginine into these two metabolites. However, the amount of citrulline produced through the NOS pathway appears lower than the amount of citrulline produced through the arginase/CPS I pathways (Blachier et al. 1991a).
Metabolism of arginine in pig enterocytes has been studied in growing piglets and has revealed that the metabolism of arginine is orientated towards synthesis in neonatal and suckling piglets, but then is orientated towards degradation up to the weaning period (Wu and Knabe 1995; Blachier et al. 1993). Indeed, at birth, several amino acids are used by enterocytes as precursors for arginine synthesis. Thus glutamine, proline, and citrulline can be used as precursors for the net synthesis of arginine in neonatal enterocytes (Wu et al. 1994; Wu 1997; Blachier et al. 1993; Flynn and Wu 1996; Wu and Knabe 1995). Interestingly, the arginine content in sow’s colostrum and milk proteins (and as free arginine) is relatively low when compared with the other amino acids, with glutamine and proline being very abundant in porcine milk (Wu and Knabe 1994; Davis et al. 1994). Thus, it has been proposed that the metabolic capacity of the absorptive epithelial intestinal cells to synthesize arginine would compensate for the relatively low content of this amino acid in milk protein (Wu and Knabe 1995), and would thus contribute to the endogenous supply of this amino acid in a context of piglet rapid growth.
Accordingly, the arginase activity in enterocytes is very low but increases slightly in the suckling period to a relatively high level in weaned animals (M’Rabet-Touil et al. 1996; Wu 1995). Also, the NOS activity is very low in enterocytes of neonatal piglets, but increases progressively up to the period of weanling (M’Rabet-Touil et al. 1993). The production of NO from arginine in enterocytes isolated from weaned pigs can be increased by the production of NADPH (a cofactor used for NOS catalytic activity) from d-glucose in the pentose phosphate pathway (Blachier et al. 1991a). In addition, although the activity of ornithine decarboxylase, the enzyme which allows the synthesis of putrescine and CO2 from ornithine, is present at a low level in enterocytes at birth, this activity falls down to a value close to the limit of detection in suckling and weaned animals (Blachier et al. 1992).
What can be the physiological consequences of these metabolic changes during piglet development? Firstly, this low arginine catabolism in enterocytes will spare a conditionally essential amino acids in a context of limited alimentary supply and de novo synthesis. Then, the increase in NO biosynthesis capacity by enterocytes from birth to weaning, although representing a low arginine utilization, is likely related to the emergence in the suckling period of different NO-dependent physiological processes including the protection of the gastrointestinal mucosa (Stark and Szurszewski 1992; Miller et al. 1993; Quintero and Guth 1992; Konturek et al. 1992; MacKendrick et al. 1993); the regulation of the intestinal motility (Calignano et al. 1992; Hata et al. 1990); and the modulation of the intestinal epithelial permeability (Kubes 1992, 1993). In newborn piglets, the low ornithine decarboxylase activity would contribute to the endogenous pool of putrescine produced from ornithine in enterocytes. However, from measurement of the capacity of enterocytes isolated from neonatal piglets for polyamine (putrescine, spermidine, and spermine) uptake, it appears that most of the intracellular polyamines likely originate from extracellular sources (Blachier et al. 1992). These sources include the polyamines present in the intestinal luminal content. Interestingly, since sow’s milk contains relatively high concentration of spermidine (Kelly et al. 1991) and spermine (Motyl et al. 1995), these polyamines may represent a significant source for piglet’s enterocyte intracellular content. In the piglets, growth of the intestinal mucosa is very intense during the first days after birth (Widdowson et al. 1976; Klein and McKenzie 1983b) and several experimental works have established that polyamines are central for the intestinal mucosal growth (Wang et al. 1991; Ginty et al. 1989). Finally, the synthesis of arginine from ornithine and the synthesis of ornithine from arginine demonstrate the existence of an intracellular arginine–ornithine cycle in suckling piglets (Blachier et al. 1993; Wu 1995).
The results obtained in vitro using enterocytes isolated from neonatal piglets which showed that these cells are orientated towards arginine production from various precursors including glutamine were complemented by in vivo experiments using suckling piglets treated with gabaculine, i.e., an inhibitor of ornithine aminotransferase. This inhibitor decreases the sequential intestinal conversion of glutamine to arginine. Using this experimental design, it was possible to demonstrate that the intestinal production of citrulline plays an important role in the endogenous synthesis of arginine and thus in its homeostasis in neonatal piglets (Flynn and Wu 1996). In these animals, proline is able to ameliorate arginine deficiency during enteral but not parenteral feeding, pointing out that the gut likely acts as an important player for the whole-body arginine supply in piglets by using proline as a substrate for arginine biosynthesis (Brunton et al. 1999; Wu 1997). The synthesis of arginine from proline may be mitigated by the plasma concentration of lactate (Dillon et al. 1999). Indeed, in enterocytes isolated from suckling piglets, lactate is able to inhibit citrulline and arginine synthesis from proline via an inhibition of proline oxidase, i.e., the enzyme responsible for the conversion of proline into delta1-pyrroline-5-carboxylate (Dillon et al. 1999). Although the factors responsible for the developmental changes in arginine metabolism in enterocytes remain unclear, it has been demonstrated that glucocorticoids play an important role in mediating the enhanced catabolism of arginine during weaning (Flynn and Wu 1997).
In pig colonic epithelial cells, much less studies have been performed regarding arginine metabolism. Amino acids from the luminal origin are not believed to be absorbed to any significant extent through the pig colonic epithelium except for a short period after birth (Smith and James 1976). Then, in contrast to the situation found in the small intestine enterocytes, which import amino acids from both luminal and blood origins, colonic epithelial cells import amino acids almost exclusively from arterial blood for metabolic and physiological purposes. l-arginine metabolism in pig colonocytes has not received much attention. Nonetheless, absorptive colonic epithelial cells isolated from the proximal colon of weaned pigs are characterized by a higher NOS activity when compared with the activity measured in enterocytes isolated from the jejunum. In contrast, arginase activity was found to be similar between pig colonocytes and enterocytes (M’Rabet-Touil et al. 1993). In the large intestine lumen, arginine released from proteins and peptides which are transferred from the ileon to the caecum may presumably serve as a precursor for the synthesis of agmatine, putrescine, and nitric oxide by the microbiota (Blachier et al. 2007). However, to the best of our knowledge, there is no published data on the production of these bacterial metabolites in the pig colonic lumen.
5.4 Metabolic Fate of Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) in Pig Enterocytes
In milk-fed piglets, 32 % of leucine in the diet are extracted by the portal-drained viscera in the first pass, with 21 % of the extracted leucine being utilized for the intestinal mucosal protein synthesis (Stoll et al. 1998). Overall, it has been estimated that 44 % of total branched-chain amino acids (BCAAs) are extracted by first-pass splanchnic metabolism in neonatal piglets (Elango et al. 2002). The catabolism of BCAAs in enterocytes isolated from developing piglets has been studied. In these cells, BCAAs are extensively transaminated and between 15 and 50 % of decarboxylated branched-chain alpha-ketoacids are oxidized depending on the age of piglets (Chen et al. 2002). Enterocytes isolated from postweaning pigs also actively degrade BCAAs (Chen et al. 2002). Other essential amino acids (i.e., histidine, lysine, methionine, phenylalanine, threonine, and tryptophan) are apparently less catabolized in developing piglet enterocytes (Chen et al. 2002, 2009) and in weaned pigs (Chen et al. 2002, 2007). Metabolism of essential amino acids by colonic epithelial cells remains to be determined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


