Fig. 3.1
SEM micrograph of duodenal mucosa on day 0 (at birth), day 3, day 7, day 21, and day 38 after birth in neonatal piglets (original images from J Physiol Pharmacol. 2005, 56 Suppl 3:71–87)

Fig. 3.2
SEM micrograph of jejunum mucosa on day 0 (at birth), day 3, day 7, day 21, and day 38 after birth in neonatal piglets (original images from J Physiol Pharmacol. 2005, 56 Suppl 3:71–87)
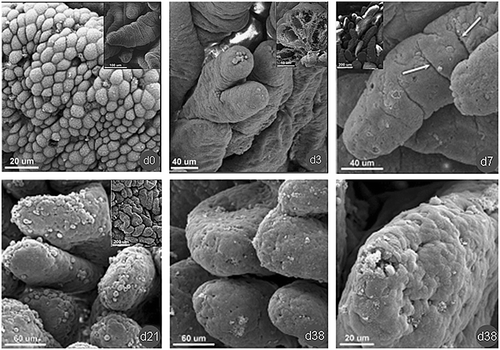
Fig. 3.3
SEM micrograph of ileum mucosa on day 0 (at birth), day 3, day 7, day 21, and day 38 after birth in neonatal piglets (original images from J Physiol Pharmacol. 2005, 56 Suppl 3:71–87)
Suckling Period
The weaning of piglets usually takes place between 3 and 4 weeks of life (Boudry et al. 2004; Zabielski et al. 2008; Zhang et al. 2012a). During the sucking period, there is an intense rebuilding of small intestine, and the most intensive processes are noted in the epithelium (Boudry et al. 2004; Zabielski et al. 2008). In duodenum, although the architecture of villi on day 7 after birth is similar to that at birth, the transversal furrows are much shallower and less numerous than at day 0. Moreover, the density of villi decreases by 58 % and 8 % compared with birth and day 3 of life, respectively. At day 14, villi are of irregular shape and the villi density is significantly decreased (Skrzypek et al. 2005, 2010). At the day 21 after birth, although most of villi are finger-shaped, a few twin-shaped villi or villi with longitudinal indention are present. Corresponding to the increase in villi length, the number of transversal furrows is further reduced on the surface of villi. In addition, the apex of duodenal villi is not smooth any more since the presence of deep, knife incision-like, furrows and “cell packets” are apparent. These “cell packets” are made of groups of enterocytes that underwent apoptosis (Skrzypek et al. 2005, 2007b, 2010; Boudry et al. 2004; Fig. 3.1). In the jejunum, the shape of villi at day 7 change from finger-like to flat and tongue-like with the transversal furrows being located on the entire villi. Moreover, the thickness of mucosa significantly decreased and the crypt depth increased by 81 % in comparison with that measured at birth. At day 14, the depth of crypt further increased and achieved the highest value (Skrzypek et al. 2010; Fig. 3.2). On the 21st day of life, the shape of most of the jejunal villi changed to wide tongue-like; and the depth and total numbers of transversal furrows is further reduced. The villus tips are still containing numerous goblet cells. In ileum, the villi on the day 7 are of finger-like, leaf-like and tongue-like shapes, and the villi surface is relatively smooth but still with some transversal furrows. On day 21 after birth, the mucosa is covered by villi with a great diversity in shape, and the villi surface is shed by numerous cells including a large number of goblet cells (Skrzypek et al. 2005, 2007b, 2010; Fig. 3.3).
Weaning Transition
During weaning transition, piglets undergo nutritional, environmental, and psychological stresses, with concomitant marked changes in the structure of the small intestine. The most marked structural changes of the small intestine during weaning transition are a reduction in villus height and an increase in crypt depth (Pluske et al. 1997; Fan et al. 2004). Following weaning at day 21, the villus height of piglets reduces to 75 % of the preweaning value at 1 day after weaning, and further declines to approximately 50 % of the preweaning value at 5 days after weaning (Hampson 1986). Crypt depth of duodenum, jejunum, and ileum also increases with age after weaning (Gu et al. 2002; Kelly et al. 1991; van Beers-Schreurs et al. 1998). Associated with the reduction in villus height and the increase in crypt depth, the morphology of the villi are also changed. In duodenum of weaned piglets (38 day old), the villi change from finger-like to leaf-like, with a wide irregular base with no transversal furrows. At that time, few micrometer spaces, on the top of villi, between the epithelial cells as well as partly broken cells are observed (Skrzypek et al. 2005; Fig. 3.1). In jejunum, following weaning on day 38 after birth, the mucosa become thinner, and the villi are of various shapes: tongue-like and fold-like with predominant incomplete division projections. In addition, no transversal furrows are observed, and there are numerous epithelial cells shed along the villi surface (Fig. 3.2). In the ileum of weaned piglets, the villi are dominated by tongue-like shapes. However, single finger-like and leaf-like villi as well as incompletely divided villi are still present. On the surface of the villi, the transversal furrows are not observed, and numerous shed cells are observed in the apical region (Skrzypek et al. 2005; Fig. 3.3). In addition, the structural changes in the small intestine are affected by the age of weaning. The changes in the piglets weaned at 14 days of age are more conspicuous than those weaned at 21 days of age (Pluske et al. 1997).
3.2.2 Epithelial Cells Differentiation Along the Crypt–Villus Axis
The maturation of the intestinal epithelium is completed at the beginning of adulthood, which forms a complex equilibrium system with multiple cell types (Cheng and Leblond 1974a, b; Mutoh et al. 2002). This equilibrium system undergoes continual renewal that involves highly coordinated processes of cellular proliferation, lineage-specific differentiation, migration, and apoptosis along the crypt–villus axis (CVA) (Gordon and Hermiston 1994). The epithelial cells differentiation along the CVA is observed during gestation. In rodent intestine, it is firstly apparent between day 17 and day 18 of gestation, when the endoderm is converted to an epithelial monolayer overlying nascent villi (Clatworthy and Subramanian 2001; Mathan et al. 1976). Stem cells located near the base of crypts give rise to progenitor cells, which expand through rapid proliferation before undergoing cell cycle arrest and ultimately differentiation into four principal cell lineages: absorptive enterocytes, goblet cells, endocrine cells, and Paneth cells (Clatworthy and Subramanian 2001). The fate of epithelial cells depends on the direction of their migration. After division, some cells go down to the bottom of the crypts and are transformed into Paneth cells with a life-time averaging 20 days. Most of cells migrate up the villus and differentiate into enterocytes, goblet cells, and endocrine cells. These cells are ultimately shed into the intestinal lumen every 3–5 days (Karam 1999). Microarrays results also establish that markers of enterocyte and goblet cell differentiation are maximally expressed in villus cells, whereas Paneth cell markers are up-regulated in crypt cells (Mariadason et al. 2005). Many signaling pathways and transcription factors with regulating functions upon small intestine cell maturation are identified. The known signaling pathways that are implicated in the regulation of cell fate determination and lineage specification in the intestine include Wnt–beta-catenin–TCF (Korinek et al. 1998; Mariadason et al. 2001; van de Wetering et al. 2002), BMP-TGF-beta-SMAD (Batts et al. 2006; He et al. 2004), Notch and its downstream factors HES1 and Atoh1 (Yang et al. 2001), and hedgehog signaling (van den Brink et al. 2004). Many transcription factors, including cdx-1 and cdx-2, kruppel-like factor 4, GATA4, 5, and 6 (Gao et al. 1998), and several forkhead family members (Burgess 1998; Clatworthy and Subramanian 2001; Shie et al. 2000), have also been suggested to be involved in the regulation of the intestinal cell maturation. Some of these transcription factors represent downstream targets of the different signaling pathways. Moreover, integrin-mediated cell-substratum and E-cadherin-mediated cell–cell adhesion, chemotactic gradients, as well as a lot of cytokines, hormones, and growth factors, have also been involved in the regulation of intestinal cell maturation (Burgess 1998; Kedinger et al. 1998). Lastly, microarrays and proteomic analysis suggest that intestinal cell maturation is primarily regulated at the transcriptional level according to the significant correlation between proteomic changes and corresponding gene expression changes along CVA (Chang et al. 2008).
3.3 Functional Development
The ontogeny of the intestinal mucosa function is discussed in Chap. 1 (Development of digestive glands in pigs) and Chap. 4 (Terminal digestion of polypeptides and amino acid absorption by the pig intestine epithelial cells during development). This section mainly focuses on the functional development of mucosa along CVA. Epithelial cells differentiation CVA is accompanied by its functional specialization. There are four approaches in studying the differentiation-dependent expression of enterocyte function in vivo, including serial sectioning technique, quantitative immunohistochemical analysis, quantitative cytochemical analysis, and sequential cell isolation in combination with biochemical and biomolecular analysis (Smith 1985). Among the four approaches, the first three approaches are difficult to quantify and limited by availability of specific antibodies. Sequential cell isolation in combination with biochemical and biomolecular assays is a useful approach to analyze digestive enzymes and nutrient transporters activities in differentiating enterocytes under various conditions. With this approach, Raul et al. (1977) analyzed the activities of alkaline phosphatase, enterokinase, aminopeptidase, sucrase, amylase in villus and crypt cells of normal rats from 5 days after birth until 8 weeks. These authors found out that the activities of enterokinase and alkaline phosphatase were always located in the upper villus during postnatal development; whereas aminopeptidase and sucrase activities appeared in the crypt cells after birth and then rose to villus during the fourth week of life. The activity of amylase was located along the entire CVA during the first 5 days of life, and then reached its maximum activity in crypt. However, after the fourth week, the maximum activity was detected in the upper villus. Furthermore, Rowling and Sepúlveda (1984), by using sequential cell isolation in combination with biochemical analysis, found a 2–3-fold increase in the number of Na+-pumping sites accompanying cell differentiation in rabbit jejunal epithelium. In neonatal pigs, Fan et al. (2001) examined the activities of alkaline phosphatase, aminopeptidase N, sucrase, lactase, and Na+/K+-ATPase along the crypt–villus using sequential cell isolation in combination with biochemical analysis. The activity of alkaline phosphatase increased quadratically during the enterocyte differentiation along the CVA in both the proximal and the distal small intestine. In addition, aminopeptidase N and sucrase activities showed a linear pattern of increase accompanying enterocyte differentiation along the CVA in both the proximal and the distal segments. Moreover, lactase activity increased cubically during the enterocyte differentiation along the CVA. In enterocytes, total Na+-ATPase activity includes two components: a ouabain-sensitive Na+/K+-ATPase activity and a ouabain-insensitive Na+-ATPase activity. Both ouabain-sensitive and ouabain-insensitive Na+-ATPase activities are increased when enterocytes differentiated along the CVA in the small intestine. The ability of nutrient absorption is also altered when enterocytes differentiate along the CVA in the small intestine. The maximal transport activity of l-glutamate was increased during the enterocyte differentiation along the CVA of neonatal porcine small intestine, but the transporter affinity of l-glutamate was decreased during enterocyte differentiation (Fan et al. 2004). At the same time than the l-glutamate uptake alteration, the expression of EAAC-1, the major glutamate transporter, was increased with neonatal porcine enterocyte differentiation along the CVA; and its expression was regulated both at the transcription and translation levels (Fan et al. 2004). As opposed to EAAC1, there is a high level of maximal SGLT1 uptake activity along the CVA of neonatal porcine small intestine. Although the mRNA abundance of SGLT1 is increased during enterocyte differentiation, there is no significant difference in SGLT1 protein abundance between crypt and villus (Yang et al. 2011). Similar to SGLT1, the SLC6A19 mRNA abundance is increased during enterocyte differentiation along the CVA. However, the B0AT1 protein is evenly expressed in the epithelium along the CVA. In addition, apical maximal Na+-Gln uptake activity, which is largely modulated by B0AT1, is expressed along the entire jejunal CVA (Yang 2011). In contrast, the mRNA abundance of ASCT2, an intestinal AA exchanger, is decreased with neonatal porcine enterocyte differentiation along the CVA, but no difference in ASCT2 protein expression is observed (Buddington 1992).
3.4 Factors Influencing Intestinal Mucosa Development
The survival of animals and humans require physiological regulation of the intestinal mucosa operating as a functional unit. This contributes to the maintenance of epithelial homeostasis by forming a selective barrier to the harsh environment of intestinal lumen. The formation of this functional unit begins in the early embryo and completes at the beginning of adulthood. Moreover, the intestinal epithelium undergoes continual renewal all along life. These developmental and renewal processes are influenced by various factors, such as genetic, neural, hormonal, and dietary factors and weaning stress.
3.4.1 Genetic Influence
Intensive growth of the piglet small intestine is faster than growth of the whole organism due to intensive remodeling of the epithelium. This remodeling is regulated by genetic influence. Skrzypek et al. (2007b) compared the postnatal development of small intestinal mucosa architecture in Polish landrace/Pietrain (PP) and Duroc/Hampshire/wild boar (DHW) crossbreed piglets by scanning electron microscopy. They found differences in villi shape modification, in transversal furrows disappearance, in extrusion zone formation and in the presence of apoptotic cell packets, reflecting differences of PP and DHW piglets in mucosa structure development and renewal. With the age-related alteration in mucosa architecture, marked changes in nutrient transporters and enzymes were also observed during the suckling and weaning periods. Detailed studies of digestive enzymes and nutrient transport showed that the age-related changes in enzymes and nutrient transporters are genetically programmed and little affected by diet or hormones (Henning 1980; Leeper and Henning 1990; Nanthakumar and Henning 1993; Toloza and Diamond 1992). Moreover, molecular biology analysis showed that the ontogenic mechanisms involved in intestine apical fructose transporter GLUT5 expression and function are independent of dietary signals (Davidson et al. 1992; Shu et al. 1997). Although the development and renewal of mucosa can be reprogrammed by interactions of genetic determinants with other factors, it is ultimately controlled by transcription regulation via multiple transcriptional elements with activatory or repressive roles (Traber and Silberg 1996).
3.4.2 Neural Influence
The enteric nervous systems (ENS), a large network of neurons and glial cells located along the gastrointestinal tract, provide an intricate network for the reflex control of intestinal mucosa (Pácha 2000). In prenatal animals and humans, the nutrients and biologically active substances involved in intestine development are mainly transferred from the mother via placenta. The contribution of ENS is presumably small but increases with fetal development (Zabielski 2007). However, in adults, almost all main gut processes such as secretion, absorption, immune responses, blood flow and complex motility patterns (such as mixing, peristalsis and migrating motor complexes) are regulated or controlled by the ENS (Burzynski et al. 2009). In comparison with adults, virtually nothing is known about the possible involvement of the nervous system in mediating mucosa structure and function development. Nevertheless, it has been suggested that the ENS function was different between early postnatal development and adulthood. Evidences suggest that ENS is involved in the regulation of the development of intestinal motility, and maybe also other function of mucosa (Zabielski 2007; Burns et al. 2009).
3.4.3 Hormonal Influence
The role of hormones in intestinal mucosa development was studied more intensively than that of ENS. A large number of hormones and cytokines have been shown to affect intestinal mucosa development and nutrients transport (Zabielski 2007). The hormones of the IGF family, including insulin, insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF-II), showed positive effects on intestinal mucosa development and intestinal adaptation (Ben et al. 2010; Lund 1998; Lemmey et al. 1991). Treatment with oral insulin significantly increased enterocyte proliferation, and decreased cell apoptosis, in rats (Ben et al. 2010). IGF-I was also reported as able to enhance crypt cell migration and cell proliferation (Liao and Lönnerdal 2010; Chen et al. 1999). Moreover, transforming growth factor alpha, epidermal growth factor, and hepatocyte growth factor are also able to promote crypt cell proliferation as measured by the 3H-thymidine incorporation assay (Nishimura et al. 1998; Sheng et al. 2006). In recent years, many newly discovered hormones or regulatory peptides have been also reported as able to regulate intestinal mucosa development. Leptin, whose receptor is widely distributed in the small intestine mucosa, has been shown to enhance small intestinal length and mitotic index, and to reduce the percentage of vacuolated enterocytes in the small intestine of neonatal piglets (Słupecka et al. 2005). Ghrelin, a growth-hormone-releasing acylated peptide, is able to delay piglet intestinal mucosa development by reducing the length of intestinal villi and increasing crypt depth (Kotunia et al. 2006). Glucagon-like peptide-2 also involves in the stimulation of stem cell proliferation and in the simultaneous reduction of the programmed cell death in neonatal piglets, thus acting as an important regulator of growth and maturation of the small intestinal mucosa (Burrin et al. 2005).
3.4.4 Dietary Influence
The compounds in diet not only play a nutritional role but also directly stimulate the growth and development of the intestinal mucosa (Buts et al. 1993; Yao et al. 2011; Kong et al. 2012b). The alterations in dietary input (amniotic fluid, maternal milk, postweaning diet) during the ontogeny of the intestinal mucosa impose different functional demands in relationship with its structure (Buddington 1994). Similarly, amniotic fluid, maternal colostrum and milk contain many biologically active substances that stimulate the development of the intestinal mucosa (Weaver et al. 1988; Xu 1996). Although the family of biologically active substances in milk is continuously increasing, EGF, IGF-I, glucocorticoids, and insulin were confirmed as compounds with important roles in stimulating epithelial cells proliferation and differentiation (Burrin et al. 1996; Buts et al. 1997; Houle et al. 1997; Xu 1996; Yeh et al. 1991). The importance of nutrients in amniotic fluid for mucosa development, and the ability of the fetal intestine to absorb exogenous nutrients were confirmed by infusing galactose into amniotic fluid of rabbits. This infusion increases fetal mucosal weights and total intestinal capacities to transport aldohexoses (Buchmiller et al. 1992). Although milk is the principal source of nutrients for most suckling mammals, the composition of milk is different between species (Jenness and Sloan 1970). In addition, the regulatory role of maternal milk in intestinal mucosa development is not only dependent on the content of nutrients and non-nutrient components, but also depends on the gradual alteration of the milk composition (Keen et al. 1981). The milk for rat pups exceeds the demand during the first week of life, whereas it becomes the limiting factor during the second week. The transition from milk to a solid diet results in significantly shorter villi and deeper crypts in the small intestinal mucosa of swine. A variety of nutrient deficiencies can impair animal growth after weaning and some of these deficiencies preferentially target intestinal mucosa development (Williams et al. 1996). The villi heights of rat pups are smaller in all segments of the intestine when there dams were subjected to protein deficiency (Subramoniam 1979).
3.4.5 Weaning Stress
Weaning is one of the most significant event in the pig life, as it corresponds to a transition from milk, which contains highly digestible protein, fat, and lactose, to dry and less-digestible starch-based diet. This transition results in reduced energy intake for the maintenance of the epithelial structure (Kim et al. 2012). Moreover, weaning of piglets coincides with, or is preceded by, the appearance of adults hydrolytic and transport characteristic of adults, and is accompanied by increases in the circulating concentrations of hormones and cytokines which are involved in the intestinal development (Buddington 1994). In response to weaning, the small intestinal mucosa of piglets undergoes major changes in structure and function (Pluske et al. 1997; Xu et al. 1996). The most obvious structural changes are the reduction in villus height and increase in crypt depth, changes which are suspected to result from the increased rate of cell loss and the increase in crypt cell proliferation (Pluske et al. 1997). It has been shown that villus height is reduced to 75 % of the preweaning value one day after weaning; and then further declines to approximately 50 % of the preweaning value 5 days after weaning (Hampson 1986). Along with the reduction in villus height and increase in crypt depth, the villi morphology also changes from long finger-like projections to leaf- or tongue-like structures (Cera et al. 1988).
References
Batts LE, Polk DB, Dubois RN, Kulessa H (2006) Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn 235:1563–1570PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


