Fig. 6.1
Bacterial cells density and major bacterial species for digesta in the different site along the digestive tract of pig
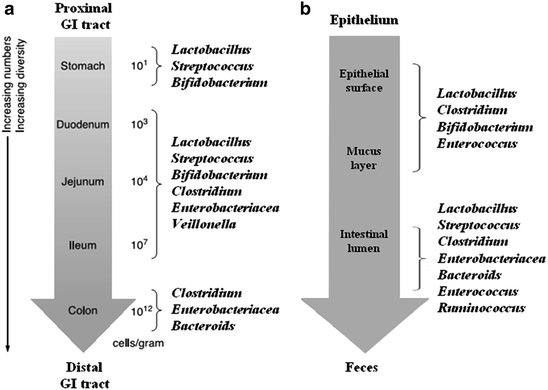
Fig. 6.2
(a) Bacterial cells density and major bacterial species for digesta in the different site along the digestive tract of pig; (b) Bacterial cells density and major bacterial species for epithelium in the different site along the digestive tract of pig
6.2 The Development of the Gut Microbiota in Piglet
The piglet is axenic in the sow uterus, and is suddenly plunged at birth into a complex bacterial environment. During its passage through the maternal vagina, the piglet is in contact with bacteria for the first time, and then meets the enormous bacterial population from the sows’ feces and rearing environment. Thus, the newborn piglet is very likely to meet potentially pathogens, and accordingly, the survival of the piglet depends on the very early establishment of defense systems. Therefore a fast and stable establishment of the gut microbiota will play a very important role in keeping the health and growth of the piglets.
6.2.1 The Nature of the First Established Strains
The establishment of the microbiota is very rapid in the piglet digestive cavities. Only few bacteria are enumerated at the third hour of life. However, after 10–12 h, the population density reaches 108–109 bacterial cells per gram in feces. After 24 h, the piglet already possesses a dominant microbiota established at a level which will not change until weaning (Ducluzeau 1983). There are differences in the order of implantation of the main bacterial groups according to the animal species. In the young mouse, facultatively anaerobic bacteria appear very early; and strictly anaerobic bacteria, which will eventually become dominant, will appear later at the third week after birth (Ducluzeau 1983). The opposite is observed in the young hare and rabbit (Ducluzeau et al. 1975). The piglet represents an intermediate case, with a simultaneous establishment from the first day of both facultatively anaerobic bacteria (mainly Lactobacillus, Streptococcus, and Escherichia) and strictly anaerobic bacteria belonging to Clostridium, Bifidobacterium, Fusobacterium, Peptostreptococcus, and sometimes Bacteroides genera (Wilbur et al. 1960; Pesti 1962).
6.2.2 Origin of the Piglet Gut Microbiota
During the process of birth, the newborn piglet meets successively several different microbial ecosystems: the sow vagina, the sow feces, and the rearing environment. It may be considered a priori that each of these ecosystems is liable to contribute to the creation of the piglet gut microbiota. In fact, the newborn piglet is provided with powerful selective systems allowing only some bacteria species to develop among all the bacteria. The first bacteria which become established in the digestive tract actually come from the sow or from the environment, but they are not the most abundant in the ecosystems met by the piglet.
6.2.3 Mechanisms Controlling the Establishment of the Gut Microbiota in Piglet
One of the main factors liable to account for the selection exerted by the newborn piglet among all the bacteria met is its dietary regime. Within 7 days after birth the piglet receives the mother’s milk as feed, which is favorable to the growth of some bacteria and unfavorable to others in the piglet gut. For example, it is observed in the piglet born to gnotobiotic sow harboring only Escherichia coli and Shigella flexneri that these bacteria are implanted in a high number after 12 h (Hayashi et al. 2002). Conversely, when the sow harbors strictly anaerobic bacteria such as bacteroides and Clostridium, these bacteria are detected in the piglet between 10 and 15 days of age, i.e., when the piglet begins to consume solid food in addition to milk. To take another example, many of Lactobacillus sp. and Lactobacillus salivarius are implanted in the piglet ileum when the piglet receives liquid milk. However, after weaning these bacteria gradually decrease, and Streptococcus alactolyticus as well as Streptococcus hyointestinalis increase if the piglets receive solid food (Wang et al. 2009).
The immune status of the sow, which affects the composition of the colostrum given to the piglet, might also influence the order of the establishment of the bacteria in the piglet intestine. Colostrum, undoubtedly, plays a role in the fight against microbial infection of digestive origin, but it has never been demonstrated that it has an effect on the microflora in the gut lumen. Immunization of the sow against different bacterial species did not prevent the further establishment of these bacteria in the piglet (Ducluzeau 1983; Hooper and Macpherson 2010). Ducluzeau studied the establishment of the fecal flora between birth and 48 h of age in a litter of conventional piglets divided into two groups: in the first one the animals were left with their dam and suckled colostrums and milk, while in the other, animals were taken off at birth, were housed in a cage inside the dam’s pen, and received sterile cow’s milk. Despite these experimental differences, no difference was observed in the systematic order of implantation of the first bacteria between both groups of piglets (Hooper et al. 2002).
Bacterial interactions certainly play an important role in the order of establishment of the piglet gut microbiota. The early development of some bacteria in the digestive tract prevents proliferation of other bacteria. A strain of E. coli is capable to eliminate from the digestive tract a strain of Lactobacillus casei derived from a commercial preparation and previously established in the axenic newborn (Dethlefsen et al. 2006). In human newborn it was shown that the inoculation of a strain of E. coli without plasmid within 2 h following birth led to the elimination or to a marked decrease of the E. coli population carrying plasmids of resistance to antibiotics, while these strains were frequently dominant in the control.
In the piglet, many studies indicate that single supplement with Lactobacillus in diet decreases the number of E. coli in the gut lumen, and concomitantly decreases the diarrhea rate among animals (Konstantinov et al. 2008).
The order of establishment of the bacteria in the piglet intestine is probably important for its survival. Many studies have shown that the supplement with antibiotics in the diet enables to increase the rate of survival in piglets and to decrease diarrhea episodes due to toxicogenic bacteria such as Clostridium and E. coli (Rohde et al. 2009). However, the use of antibiotics may result in the formation of resistant bacteria. In addition, the disturbance by the supplement of antibiotics of the initial establishment of the gut microbiota may lead to some negative effect in the piglets like an impairment of the piglet immune response, thus producing cross infection. The practical application of the bacterial interaction concept was found to be more useful in the above case than the use of antibiotics (Rohde et al. 2009). If the young hare are inoculated within the hour following birth with a complex flora derived from a healthy young hare, the animal is totally protected from neonatal diarrhea without further pathological manifestation at weaning. This protective flora, preserved in gnotobiotic mice, has never been simplified up to now to obtain a mixture of pure strains liable to be cultured in vitro (Ducluzeau et al. 1981a, 1981b). A protective effect was also demonstrated in the axenic newborn piglet: the early inoculation of a selected strain of E. coli from pig origin was found to protect the animals from the further development of toxinogenic strains of E. coli provided that the latter were not capable of adhering to the gut mucosa (Duval-Iflah et al. 1983).
6.2.4 Lactobacilli Community Development
There are several hundred bacterial species in the piglet GI tract. All the members of the piglet gut microbiota are needed for the gut to develop its specific intestinal functions (Hooper and Gordon 2001). However, Lactobacilli are the most prevalent bacteria, and are often considered beneficial because they are important for maintaining a healthy and stable microbiota in the GI tract. It has been postulated that Lactobacilli has several health-promoting effects, including immuno-stimulation, alleviation of food intolerance and allergy, and prevention of diarrhea and intestinal infection (Servin 2004; Bengmark 2000; Salminen et al. 1998). Consequently, a normal Lactobacilli community development in time and space in the gut is significantly important for the health and growth of the piglet.
The experiments conducted by Yao et al. analyzed Lactobacillus diversity and development of neonatal piglet gut microbiota during the first 4 weeks of life by a combination of denaturing gradient gel electrophoresis (DGGE) and 16S ribosomal DNA sequence (Fig. 6.3a). The bands in DGGE profile represent the majority of the dominant Lactobacillus populations in the community, and their appearance and disappearance reflect approximate changes in the Lactobacillus community composition. The intensity of a band provides a rough estimate of the proportion of the corresponding population in a sample. The Lactobacillus communities in each compartment changed from simple to complex and returned to simple during this 4-week period. The most complex banding pattern appeared at week 3. There were several new bands (or new lactobacilli) only observed at this time, and disappeared again at week 4 (Yao et al. 2011).
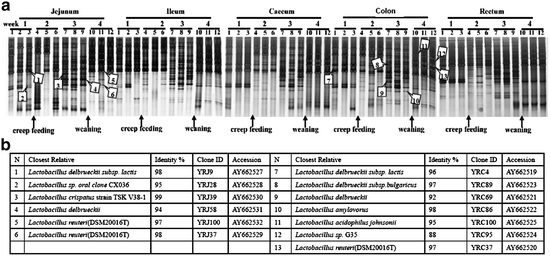

Fig. 6.3
(a) Succession of the Lactobacillus community of digesta during the first month in the piglet intestinal tract. Changes of feeding strategy are indicated by arrows. The bands identified from the 16S rDNA clone libraries are numbered and are indicated by arrowheads. (b) Closest relative as determined by comparative sequence analysis, identity with this relative, clone ID, and accession number for each band identified in (a)
Yao et al. also compared the mobility of the cloned fragments with the mobility of fragments amplified from the original samples by DGGE, and the DNA sequences of clones that corresponded to dominant bands were determined (Fig. 6.3b). Six dominant DGGE bands generated from jejunal digesta were matched with sequences that show 94–98 % similarity to those derived from L. reuteri, L. delbrueckii, and L. crispatus. Seven dominant DGGE bands generated from colon digesta were matched with sequences that show 88–99 % similarity to those derived from L. reuteri, L. delbrueckii, L. amylovorus, and L. acidophilus. Amplicons related to L. reuteri were found in all DGGE fingerprints from jejunal digesta from 1-, 3-, and 4-week-old animals. Amplicons related to L. amylovorus were present in all DGGE fingerprints from colon digesta from 1-, 3-, and 4-week-old animals. Amplicons related to L. delbrueckii were found before weaning, L. crispatus in the stage of creep feeding, and L. acidophilus after weaning (Yao et al. 2011). In the experiments conducted by Wang et al., L. reuteri and L. amylovorus were the dominant Lactobacilli species in the ileum in both nursing and weaned piglet (Wang et al. 2009).
In conclusion, Lactobacillus communities follow successional changes associated with piglet age and diet shifting. Creep feeding stabilizes the Lactobacillus community of weaning piglets. Within the Lactobacillus community, some members like L. reuteri and L. amylovorus/L. sobrius might be permanent colonizers, while L. delbrueckii, L. acidophilus, L. salivarius, L. mucosae, and L. crispatus might be transient members of the Lactobacillus communities in the piglet’s GI tract.
6.3 Factors Influencing the Composition of Piglet GI Tract Microbiota
As said above, the piglet is born without microorganisms. Starting at birth, the gut microbiota must develop from a simple unstable community into a complex community, which comprises microorganisms (bacteria, archaea, fungi, viruses, and protozoa) that represent superior competitors for a complex intestinal ecosystem (Palmer et al. 2007). It is generally considered that the genotype of host and the diet are the major factors defining a microbial intestinal niche. However, many other factors also influence mammal intestinal microbiota such as interactions between individual colonizers, age, weaning, as well as transient microorganisms (pathogens and probiotics) (Deng et al. 2007; Yin and Tan 2010).
6.3.1 The Genotype of the Piglet Influences Gut Microbiota
The genotype of the piglet is considered as a factor influencing variation of gut microbiota between individuals. Differential action of genes that control the immune system is involved in such an effect (Ley et al. 2006). For instance, the piglet immune system modulates the intestinal microbiota composition by restricting microbial penetration through the host mucosal barrier, and by secreting different antimicrobial products such as peptides, as well as antimicrobial enzymes (Cobb et al. 2004; Macdonald and Monteleone 2005). The genotype of the piglet determines the availability of specific attachment sites and host-derived resources, which influences the intestinal microbiota. So, monozygotic twins exhibit fewer differences in the intestinal microbiota than their unrelated marital partners. However, there are small differences between the intestinal microbiota of the monozygotic twins, suggesting that maternal transmission (Ley et al. 2005) is another determining factor. The initial colonizing microbiota influences the eventual microbial composition of the intestine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


