Chapter 22 Dermatologic Therapy
Anatomy and Physiology of the Skin as They Relate to Drug Therapy
The skin is the largest organ of the body, accounting for 12% of body weight in the adult dog and 24% in the puppy.1 Although structurally canine and feline skin markedly varies from human skin, some similarity is maintained among the species. Generally, skin is thickest on the head, dorsum, and plantar and palmar surfaces of the feet; thinner on the ventral abdomen, medial aspects of the limbs, and inner pinnae; and thinnest on the scrotum.2 The skin is perforated by several appendages, the number and structure of which varies among the species. In cats and dogs, these include hair follicles, sebaceous and sweat glands, and nails.
Histologically, the skin is composed of the epidermis and dermis. Dermis is essentially composed of connective tissue, including collagen, elastin, and reticular fibers, and amorphous ground substance. It can be roughly separated into a dense, deeper reticular layer that connects the dermis to the hypodermis (composed mostly of fat) and a more superficial, loosely packed papillary layer. The dermis contains an arterial and venous network that provides nutrients to the epidermis and receives topically administered drugs able to penetrate this region, which distribute to the rest of the body.2 Cutaneous blood flow rates can affect percutaneous absorption of drugs. Cutaneous blood flow in the dog and cat is greatest in the skin of the ventral abdomen and pinnea.2 This fact, coupled with skin thickness, leads to these regions serving as the site of drug delivery for many topically applied drugs intended to have systemic effects.
The epidermis is composed of stratified squamous keratinized epithelium that undergoes sequential superficial differentiation. Five layers of the epidermis exist, with the stratum basale being deepest, followed by the stratum spinosum, stratum granulosum, stratum lucidum, and the stratum corneum (the most superficial layer). Among these layers the stratum corneum is the most important to topical drug therapy because it is the primary barrier. The stratum corneum consists of several layers of dead cells, which present a significant lipid barrier to drug penetration. The thickness varies with the area of the body. The cells are aligned to minimize water loss and are surrounded by a plasma membrane that serves as a barrier to movement into or out of the skin.2
The epidermis is anaerobic2 because of the absence of capillaries that directly provide oxygen to the cells.2 Despite the fact that 80% of the total energy requirements of the skin occur by anaerobic glycolysis, the skin is metabolically active. Drugs that are able to pass through the stratum corneum potentially are subjected to drug-metabolizing enzymes similar to those in the liver. The skin has a great capacity to synthesize lipids, which are located in the extracellular (intercellular) material, the primary barrier to drug penetration in this region of the epidermis.2 Lipids are important to intercellular cohesion, permeability (barrier), function, and normal desquamation of mature corneocytes.3 Epidermal lipids include both free and esterified fatty acids, sphingolipids, free and esterified cholesterol, and phospholipids.3 The lipids form a bilipid layer, with the hydrophobic and hydrophilic ends aligning within themselves.2 As the epidermis differentiates, the fatty acid component tends to increase. Alterations in the lipid layer result in the release of arachidonic acid and the subsequent formation of inflammatory mediators. Keratin is the major protein of the skin and is the foundation of the hair.2
Principles of Topical Drug Therapy
Drug Movement and the Skin
The skin functions as a barrier to prevent loss of water, electrolytes, and macromolecules and to exclude external agents (chemical, physical, and microbiologic) from the internal environment. The stratum corneum is the layer of the epidermis that is primarily responsible for this physical barrier because of the abundance of keratin and the configuration and content of the intercellular lipids. Topically applied drugs can be absorbed by three routes; they are, in order of importance or magnitude, the stratum corneum (between rather than through the cells), hair follicles, and sweat or sebaceous glands that open into the hair follicle. Movement of drug through the stratum corneum occurs by passive diffusion. Only a very small proportion of topically applied drug penetrates the stratum corneum. Despite the alignment of the lipid layer, both lipid-soluble and water-soluble drugs can pass through the stratum corneum, although passage may occur through the appendages. In general, permeability of lipophilic drugs through intact skin is generally greater than that of polar drugs.4 More drug is likely to pass through the skin of heavily haired animals because of the larger number of hair follicles.
Before a drug can move through the stratum corneum, it must first move out of the vehicle. Thus factors that affect percutaneous absorption are not limited to the drug but include factors involving the vehicle. Drug movement through the skin has been mathematically described (Fick’s law)2 to be directly proportional to the partition coefficient between the vehicle and the stratum corneum, the concentration of drug dissolved in the vehicle, the diffusion coefficient, and the surface area of the skin to which the drug is applied. Percutaneous absorption is inversely proportional to the depth of the stratum corneum (and additional layers). The driving force for absorption, as with any drug movement, is concentration of diffusible drug. The higher the concentration of the dissolved drug in the barrier (stratum corneum), the greater the diffusion “gradient.” Drugs with a high degree of lipid solubility achieve higher concentrations in the stratum corneum because it is lipophilic. Large drug molecules are absorbed less readily.
Vehicles
Characteristics of a Vehicle
The physical and chemical characteristics of the vehicle and the drug largely determine drug movement. The partition coefficient describes the relative affinity of a hydrophobic phase and a hydrophilic phase. The greater the partition coefficient, the greater the affinity between the drug and the lipid phase of the skin, which generally results in an increase in percutaneous absorption of the drug. Once the skin is penetrated, however, the drug must be able to leave the lipid phase of the skin if it is to reach systemic circulation. Drugs with very high partition coefficients tend to remain in the lipid layer, causing a reservoir effect. A partition coefficient of 1 is desired for topical medicaments.2
The rate of vehicle penetration through the stratum corneum also influences percutaneous absorption. If vehicle penetration of the stratum corneum is more rapid than penetration of the skin, the concentration of drug in the vehicle on the surface of the skin increases, perhaps to the point of precipitation, slowing absorption. Evaporation of the vehicle will cause the same effect.2 Some vehicles are used to facilitate drug movement into the skin. For example, dimethylsulfoxide (DMSO) is so hygroscopic that it readily moves through the skin, carrying many drugs with it. The vehicle may contain ingredients (e.g., Tween) intended to facilitate percutaneous drug absorption by altering the integrity of the stratum corneum. Disruption of the composition or lipid orientation of the stratum corneum enhances drug penetrability. A vehicle that hydrates the corneum facilitates drug penetration. Occlusion of the skin increases hydration; vehicles can occlude by preventing skin transpiration, the passage of water vapor from the skin. Occlusive bandages also can be used to facilitate drug absorption. Water associated with hydration alters the compact structure of the corneum, decreasing resistance to drug movement. Dehydration of the stratum corneum decreases drug absorption; rehydration might be indicated before drug application.
Types of Vehicles
Water itself can be therapeutic. Bathing with water vehicles (especially shampoos) contributes to dermatologic therapy by removing debris, including potential allergens, bacteria, and other organisms, from the skin surface and rehydrating and cooling the skin (if cool water is used).5 The addition of other drugs (shampoos, soaks, and dips or rinses) to water, forming an aqueous solution, suspension, or lotion, can create other therapeutic effects. Aqueous medications are often the topical treatment of choice for acute exudative dermatoses.
Shampoos, a type of water vehicle, can be very effective adjuvants for the control of dermatoses.5,6 In general, contact time should be at least 10 minutes. Shampoos generally are applied once to twice weekly. Examples of shampoos with therapeutic intent include hypoallergenic shampoos, which are cleansing and moisturizing; antipruritic shampoos, which often contain colloidal oatmeal along with antihistamines, anesthetics (pramoxine hydrochloride), or cortisone 1%; and insecticidal shampoos containing compounds such as pyrethrins, carbaryl, and permethrin. Application of rinses, sprays, or lotions can enhance the residual effect of shampoos.
Creams and ointments are mixtures of grease or oil and water that are blended together into an emulsion. In general, ointments are greasy to the touch and form an occlusive layer over the skin, reducing water loss. Creams are smooth to the touch and, once applied to the skin, are rapidly absorbed or evaporate (i.e., there is no occlusive layer left on the surface of the skin). In general, ointments are contraindicated in exudative areas. Examples include triple antibiotic ointments and creams and hydrocortisone ointments and creams. Hydrocarbon bases are emollient, being composed of vegetable oils and animal fats. Examples include oleic acid, paraffin, petrolatum, and wax. They generally are hydrophobic and occlusive, causing the stratum corneum to hydrate. They are greasy, however, and cannot be washed off. Anhydrous absorption bases contain little to no water but readily accept large amounts of water while maintaining a thick consistency. Examples include hydrophilic petrolatum and anhydrous lanolin.
Emulsions are oil and water combinations. Water–oil emulsion bases are water-washable bases that are easily removed from the skin surface. The oil phase generally is petrolatum with an alcohol; the aqueous phase may be water, propylene glycol, polyethylene glycol, or glycerin. Oil-water emulsion bases are composed of an aqueous phase that is greater than the oil component. These tend to be water washable, nongreasy, and nonocclusive. Finally, water-soluble–based ointments have no hydrophobic lipid base. They are completely water soluble, do not hydrolyze, and do not support the growth of microorganism contaminants in the product. If the preparation is in a gelled medium, the product is a gel (e.g., a combination of propylene glycol, propylene gallate, methylcellulose, polyethylene glycol, and others). DMSO is commonly prepared as a gel.2 Gels are clear, colorless, and water miscible. Gels are becoming more popular because they can be rubbed into the skin to completely disappear and do not leave a sticky feeling. Examples of gels in veterinary medicine are KeraSolv, OxyDex and Pyoben.
DMSO has the ability to allow some substances ordinarily unable to penetrate the skin to be carried through it. DMSO is a waste product of wood processing that has been used in a large number of topical medicaments. In addition to its hydrophilic actions, DMSO is characterized by bacteriostatic, antiinflammatory, fibrinolytic, and vasodilatory actions. Topical analgesia may reflect a thermal effect, which occurs with direct application.2 At concentrations greater than 70%, however, DMSO can cause skin irritation. In concentrations greater than 50%, DMSO has been shown to enhance the percutaneous absorption of a large number of drugs, including glucocorticoids, antibiotics, hormones, and antiinflammatory agents. Absorption increases as DMSO concentration reaches 100%.2 DMSO increases percutaneous absorption of fluocinolone (a potent glucocorticoid) by a factor of five and other compounds by as much as a factor of 25. DMSO is approved for use only in the horse (for traumatic musculoskeletal injuries) and in the dog (in Synotic, a commercial steroid ear preparation). Any other use for DMSO is considered extralabel use. Toxic effects that should be considered when DMSO is used include teratogenicity (contraindicated in pregnant animals); potential for inducing degranulation of mast cells in underlying skin; and, in cats, hemolysis with hemoglobinuria and methemoglobinuria. DMSO has been shown to induce lenticular changes in animals and humans. Rubber gloves should be worn when DMSO is handled.
Demulcents are high-molecular-weight water-soluble compounds that reduce irritation. Like protectants, they can coat the surface of damaged skin, protecting the stratum corneum and its underlying structures, and they inherently reduce irritation. Examples include mucilages, gums, dextrins, starches, methylcelluloses, and polyvinyl alcohol.2 Among those most commonly used in veterinary medicine are glycerin, propylene glycol, and polyethylene glycols. Glycerin, when used in high concentrations on the skin, can dehydrate and irritate it by increasing transepidermal water loss. Propylene glycol is miscible with water. Like glycerin, it is hygroscopic, is not occlusive, and also is bacteriostatic and fungistatic. As such, it might be considered the ideal vehicle. It spreads easily on the skin surface, has a low evaporation rate, is not greasy, and may hydrate rather than dehydrate the skin.2 A mixture of one part propylene glycol to one part water has been used to treat canine sebaceous adenitis. Topical hypersensitivity occurs occasionally. Several polyethylene glycols are available. They differ markedly in molecular weight, with the number directly correlating with size and viscosity. Polyethylene glycols that are 900 or above tend to be semihard to waxy solids at room temperature; lower-molecular-weight products are liquid. These compounds are not easily hydrolyzed but are very water soluble and nontoxic.2
Classes of Topical Drugs
Many topical products are commercially available, and many have multiple effects. Some of these agents are also discussed in other chapters (e.g., those on parasitology, antibacterials, and antifungals).
Antiseborrheics
Sulfur is keratolytic (keratolytics hydrate and soften the stratum corneum, promoting its mechanical removal) and keratoplastic (keratoplastics normalize cornification). It has a mild follicular flushing action but is not a good degreaser. It also has antibacterial and antipruritic effects. Its keratolytic effects may reflect inflammation that ultimately causes sloughing of the stratum corneum. Keratoplastic effects probably reflect cytostatic effects.2 Many commercially available products containing sulfur are available. Shampoo products containing sulfur may have additional active ingredients for enhanced therapy. Lime sulfur (LymDip) is used for its antifungal, antipruritic, and antiparasitic effects.
Benzoyl peroxide (2% to 5%) is keratolytic, bactericidal, degreasing, and follicular flushing. It also is a strong oxidizer, free radical generator (and therefore antibacterial), and antimicrobial.2 Benzoyl peroxide is metabolized by viable epidermal cells in the skin to benzoic acid. In high concentrations it can irritate the skin. It may be too drying for some patients with seborrhea sicca. These products are not well tolerated by cats and should not be used on them. Commercial products available for veterinary patients include Oxydex, Pyoben, Derma Ben SS, and Benzoyl-Plus shampoos. A gel form of 5% is available for veterinary use, primarily for treatment of chin acne.7 Other uses include fold pyodermas and local superficial or deep pyodermas. Benzoyl peroxide will bleach clothing.
Resorcinol is a keratolytic agent that also has bactericidal and fungicidal effects. It is a protein precipitant that promotes keratin hydration, acting as a keratolytic.2 It often is combined with another keratolytic (e.g., sulfur, salicylic acid).2
Retinoids
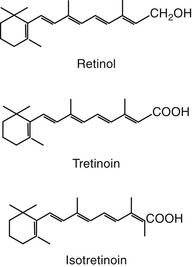
Retinoids are natural or synthetic derivatives of retinol (vitamin A) that exhibit vitamin A activity (Figure 22-1).8 Dermatologic effects of vitamin A include epithelial differentiation. Vitamin A deficiency causes metaplasia of glandular epithelia; excessive vitamin A causes keratinizing epithelia to differentiate into a secretory epithelia.9 The antikeratinizing effects are the target of drug therapy.9 Retinoids tend to “normalize” the skin. Although natural retinoids have proved to be too toxic for clinical use, the synthetic products are characterized by specific effectiveness with decreased toxicity. They tend to vary in bioavailability, in metabolism to active versus inactive metabolites, and in tissue distribution patterns. First-generation compounds include retinol and its derivatives tretinoin and isotretinoin. The second-generation products are synthetic and include etretinate and acitretin, approved for treatment of human acne and psoriasis, respectively. The third-generation compounds, arytenoids, are in development.8
The effects of the retinoids include cellular proliferation and differentiation, immunomodulation, inflammation, and production of sebum. Their actions are mediated by retinoic acid receptors, members of the thyroid/steroid receptors.8 Retinoids may influence genomic expression of cells by altering RNA synthesis, typical of other steroids. Tretinoin increases dermal thickness and granular layer thickness, decreases melanocytic activity, and increases the secretion of a polysulfated glycosaminoglycan intercellular matrix. In humans wrinkling is reduced. It is formulated as a 0.01% to 0.1% topical preparation. Therapy begins with lower concentrations and gradually increases. Adverse effects include erythema, peeling, burning, and stinging, which tend to decrease with time and are less likely to occur when the drug is prepared as an emollient.
Isotretinoin normalizes keratinization of the follicular epithelium and reduces sebum synthesis and, in human beings, Proprionobacterium acnes. It is administered orally, however, with cumulative doses being important to efficacy. Toxicity is manifested in the skin and mucous membranes; is dose dependent; and, in humans, may facilitate the growth of Staphylococcus aureus. Dermatologic manifestations include epistaxis, dry eyes, blepharoconjunctivitis, erythematous eruptions, and dry mucous membranes.8 Systemic manifestations can be minimized with short-term therapy and include increased liver enzymes, myalgia, and arthralgia. Teratogenicity occurs with all retinoids; the drugs generally are contraindicated in pregnancy. Isotretinoin was studied in dogs.10 Four of 29 developed conjunctivitis, which resolved once therapy was discontinued. Cats may have a higher incidence of side effects, including periocular erythema, epiphora, and blepharospasm. The potential veterinary applications of isotretinoin include selected abnormalities of sebum production such as primary idiopathic seborrhea and comedo syndromes.
Etretinate is a synthetic aromatic retinoid that is effective for the treatment of inflammatory psoriasis. The retinoids are highly potent aromatic analogs of retinoic acid and represent the third generation. Etretinate is extremely lipophilic and is stored in adipose tissue. Accumulation is sufficient to allow detection of the drug in humans 2 to 3 years after its use is discontinued. It normalizes keratin expression in epidermal cells, suppresses chemotaxis, decreases stratum corneum cohesiveness, and may impair cytokine function.8 It is less likely than isotretinoin to cause conjunctivitis, but hair loss, cutaneous exfoliation, bruising, and liver dysfunction are more common. Collection of a baseline minimum database is recommended for humans before its use. Its teratogenicity precludes use by women of childbearing age; owners of animals using the drug should be warned of its contraindications.8 The drug may no longer be available because of its adverse effects. Acitretin has now replaced etretinate in the U.S. market. A dose of 0.5 to 1 mg/kg orally per day was suggested.11
The use of retinoids in clinical veterinary medicine has not been well established. Animals are not afflicted by skin diseases typical of those for which retinoids are indicated in human patients (e.g., psoriasis, acne). Use is limited by lack of known effects and indications, cost, and the risk of side effects. Dogs, however, appear to be more tolerant of the retinoids than human beings.10,12 Side effects that have been reported in dogs include inappetence, vomiting, diarrhea, thirst, pruritis, conjunctivitis, cheilitis, stiffness, and hyperactivity.12 Keratoconjunctivitis has been reported in dogs.10,12 Tear composition is changed, leading to more rapid evaporation. Schirmer’s tear test should be monitored monthly for the first 6 months of therapy. Clinical pathology changes are rare, but monitoring before and 30 days after the start of therapy is recommended for dogs receiving synthetic retinoid therapy.12 In cats the most common side effect is anorexia. Teratogenicity is a likely problem, particularly with etretinate, when used for intact females.
The most common use of synthetic retinoids in dogs has been for treatment of keratinization disorders of dogs, particularly primary seborrhea of cocker spaniels. Etretinate has been evaluated in spaniels with idiopathic seborrheic dermatitis (approximately 10 mg or 0.75 to 1 mg/kg orally per day). Animals generally respond well with a decrease in scaling, a softening and thinning of seborrheic plaques, decreased pruritis, and reduction in odor. Response occurs within 2 months, and improvement continues for at least 2 more months.12 The more severe the syndrome, the slower the time to response; discontinuation of therapy is likely to result in recrudescence of clinical signs within 3 to 12 weeks.9 The drug was minimally effective in the treatment of ceruminous otitis associated with seborrhea.12 Maintenance therapy ranges from 10 mg every other day to 10 mg daily, alternating 30 days on and 30 days off. Isotretinoin (1 and 3 mg/kg per day) appears to be much less effective.9 Neither isotretinoin nor etretinate has proved to be effective in West Highland White Terriers, and etretinate was ineffective in Basset Hounds. Both etretinate and isotretinoin have proved effective in the treatment of Schnauzer comedo syndrome9,12 and canine ichthyosis. Newer applications of the synthetic retinoids include hair follicle dysplasia (etretinate), which should respond in approximately 30 days, and selected dermatologic cancers. These include solar-induced squamous cell carcinoma (etretinate 2 mg/kg divided or once daily for 6 months), mycosis fungoides (etretinate or isotretinoin 3 to 4 mg/kg divided or once daily), and selected benign cutaneous neoplasms (multiple sebaceous adenomas, epidermal cysts, inverted papillomas, and infundibular keratinizing acanthomas).12
Isotretinoin for the treatment of disorders of the sebaceous glands has been variably successful; success may be breed dependent. It was proved effective in sebaceous adenitis of standard poodles in one study but ineffective in another.9 A higher dose (2 to 3 mg/kg) has been recommended. Hair growth in poodles that do respond is abnormal, however, in that kinks are lost. Vizslas appear to respond to isotretinoin very well.9 In contrast, neither isotretinoin nor etretinate appears to be effective in sebaceous adenitis of Akitas.
Retinoids appear to be safe for cats but do not appear to be effective for solar-induced squamous cell carcinoma. Either isotretinoin or etretinate (2 to 2.5 mg/kg) can, however, be beneficial for preneoplastic actinic disease. Cats tolerate retinoids well, although anorexia is more common in cats than in dogs.12 Reducing treatment to every other day or every other week may limit this side effect. Topical tretinoin (0.025% cream) may be efficacious for treatment of feline acne. The product must be used very sparingly, however, so as not to incur severe tissue irritation.12
Ceruminolytics
Ceruminolytics are topical products that emulsify, soften, and break up waxy debris and exudate. Generally, they are detergents or surfactants used for cleaning or flushing the ear. Examples include dioctyl sodium sulfosuccinate, which is water soluble (and perhaps less messy); squalene, which is an oil-based product; propylene glycol; glycerin; and oil. Carbamide peroxide differs from most other ceruminolytics in that it is a humectant, releasing urea and oxygen to cause its foaming action. Ceruminolytics and drying products are often combined with alpha hydroxy acid such as lactic, salicylic, benzoic, and malic acids. These acids have the added advantage of decreasing local pH and are mildly antibacterial and antifungal, along with their keratolytic effects. These products need to be placed in the ear 3 to 15 minutes before flushing with water or saline.
Antipruritics
Antipruritics (topical)5 are used to provide temporary relief of itching, but their efficacy is debatable. In general, antipruritics relieve itching by four mechanisms. (1) The itching sensation can be substituted with another sensation (such as heat or cold). Examples of agents with this mechanism of action include menthol, camphor, warm soaks or baths, and ice packs. (2) The skin can be protected from external factors such as scratching, biting, irritants, and changes in humidity or temperature. This can be accomplished with bandages or impermeable protective agents. (3) Peripheral sensory nerves can be anesthetized by local anesthetics (benzocaine, lidocaine). These drugs may, however, cause allergic sensitization. A new product in this category for small animals is pramoxine (Dermacool). (4) Biochemical agents used topically to treat pruritus include glucocorticoids and antihistamines. Despite the fact that the skin contains large numbers of mast cells, topical antihistamines do not seem to be efficacious. Systemic antihistamines, on the other hand, may be useful.
Topical glucocorticoids may not be as potent as their oral or injectable counterparts.13 Like systemic glucocorticoids, however, the active ingredients vary in potency and risk of side effects. For topical glucocorticoids ointments provide greater efficacy than creams. Topical glucocorticoids can be absorbed through the skin and cause systemic effects. This is more likely to be a problem with the potent fluorinated agents (betamethasone, dexamethasone, triamcinolone, flumethasone, and flucinolone) and when combined with DMSO (Synotic). Gloves should be worn to apply these drugs. There are many forms of glucocorticoids available for topical use, including use on extensions of the skin such as the external ear canal and anal sacs. Once absorbed through the skin, topical corticosteroids are handled by the body in the same capacity as systemically administered glucocorticoids. The extent of percutaneous absorption of topical glucocorticoids depends on factors such as the vehicle, the ester form of the steroid (greater lipid solubility enhances percutaneous absorption), duration of exposure, surface area, and integrity of the epidermal barrier. A new product containing 0.015% triamcinolone (Genesis spray, Virbac U.S.) has been formulated to be applied topically to the entire skin surface for its antipruritic effect.11 This product may also be used for spot treatment of pruritic regions. Ointment bases are occlusive and are therefore more likely to increase percutaneous absorption of the same glucocorticoid in a cream base. Highly potent preparations in any form should not be used on abraded skin.
Irritants
A number of products are used to inflame or irritate the skin to various degrees. Examples include those that cause hyperemia (rubefacients), inflammation (irritants), and cutaneous blisters (vesicants). Caustics are corrosive agents that destroy tissue after one or more applications. Examples include camphor, coal tar, creosote, menthol, methyl salicylate, iodine, mercuric iodide, alcohols, and pine tar. Among these, only coal tar is used to any degree in veterinary medicine. It is a by-product of bituminous coal distillation and, as an irritant, decreases epidermal synthesis of DNA.2 Escharotics also are corrosives that precipitate proteins, causing the formation of a scab and eventually a scar. Examples include glacial acetic acid, aluminum chloride, gentian violet, phenol, salicylic acid, and silver nitrate. The uses in veterinary medicine are few.2 Irritant products have been used empirically for many centuries. Their proposed mode of action is masking of moderate to severe pain by milder pain caused by the application. Another desired effect of irritants is to induce a healing action on chronic wounds. The idea is to heal chronic inflammation by converting it to acute inflammation. Chemicals used include phenol, formalin, mercuric iodide, and camphor. Menthol-containing products are sometimes used to treat acral lick dermatitis (“lick granuloma”) in dogs but may be painful upon initial application. Capsaicin has been used topically on human beings for relieving arthritis pain. It also has been used to treat acral lick dermatitis in dogs.
Antimicrobials
Alcohols, iodine, chlorhexidine, iodophors, and hexachlorophene can be effective in the treatment of infectious skin diseases (see Chapters 10, 11, and 13).
Benzoyl peroxide, discussed with the antiseborrheics, is a potent broad-spectrum antibacterial agent. It is an excellent adjunctive treatment for pyoderma. In a clinical trial of four antibacterial shampoos (containing 3% benzoyl peroxide, 0.5% chlorhexidine, 1% available iodine in a povidone complex, and 0.5% triclosan combined with 2% salicylic acid and 2% sulfur), although each was effective prophylactically, the product containing benzoyl peroxide was most effective.14 Use of the veterinary products (as opposed to the human proprietary products) is strongly recommended. Benzoyl peroxide is very irritating to cats and should be avoided.
Mupirocin is a compound produced by Pseudomonas fluorescens that is effective against superficial (topical) infections caused by Staphylococcus species. It is less active against gram-negative organisms and in humans is not active against normal skin flora. It inhibits protein synthesis by binding to bacterial tRNA synthetase. Prepared as an ointment, it often is used for prophylaxis of superficial infections resulting from wounds and injuries.8 Veterinary products containing mupirocin recently have become available (Muricin ointment 2%, Dechra).
Antiparasitics
Drug delivery systems
Antiparasitics are available as sprays, powders, shampoos, foams, spot-ons, tablets, and dips. The use of parasiticides is discussed in Chapter 15. Spot-on products are commonly used as broad-spectrum antiparasitic agents. Their ease of use and efficacy make them a preferred product for many circumstances. Powders are the safest formulation but must be frequently applied. They often are messy and must be applied deep into the coat to be effective. Sprays may have little residual effect depending on the active ingredient and concentration of the product, and the noise made during application often frightens the animal. Efficacy can be enhanced by ensuring adequate penetration of the hair. The hair should be brushed away from the skin so that the spray can reach the skin. The face can be treated by spraying into a glove and then rubbing the face. A water-based spray may cause less drooling than an alcohol-based spray.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree