Chapter 34. Dental Health and Diet
Periodontal disease and inflammation of the gingivae are common disorders in dogs and cats. 1 Gingivitis is caused by the formation and persistence of dental plaque on the surface of the teeth. If untreated, this can progress to periodontal disease, which affects the gingivae, periodontal ligament, cementum, and alveolar bone. Periodontal disease is associated with oral pain, malodorous breath, ulceration, and the loss of alveolar bone and teeth. The bacteremia that often accompanies periodontitis may also lead to damage to other organs in the body. Although a direct causal relationship has not been proven, periodontal disease has been implicated as contributing to systemic diseases involving the kidneys, cardiovascular system, lungs, and immune system. 2. and 3. Because periodontal disease is a common and serious disorder in dogs and cat, studies have focused on nutrition and diet as risk factors for its development and as potential means for reducing gingivitis and calculus formation, preventing its progression to periodontal disease and supporting long-term dental health.
INCIDENCE AND DESCRIPTION
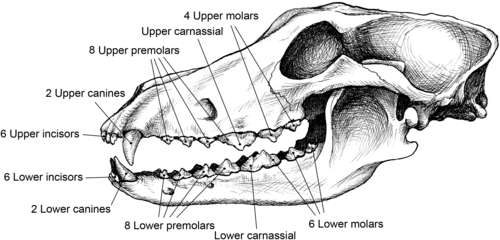
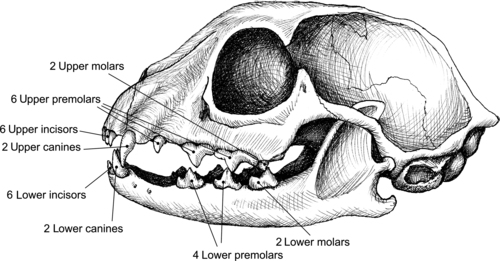
The types of dental health problems that occur in dogs and cats differ somewhat from those typically seen in humans. Because of the sharp inclined planes of their dentition, dogs and cats are not susceptible to the formation of tooth caries (i.e., cavities). In dogs, demineralization of teeth is not common because of the alkaline nature of their saliva. Cats, in comparison, can produce saliva with a more acidic pH, making tooth demineralization possible in this species. Overall, the three primary dental problems that are seen in dogs and cats are oral malodor, gingivitis, and periodontitis. The term periodontal disease typically refers to gingivitis and periodonitis together. Odontoclastic resorptive lesions in cats also are associated with gingival inflammation and periodontal disease (Figure 34-1 and Figure 34-2). 4. and 5.
 |
| Figure 34-1 |
 |
| Figure 34-2 |
Periodontal disease is one of the most common diseases observed by small-animal practitioners, and it is the most prevalent type of oral disorder. It has been reported in domestic pets for at least 80 years and is considered a worldwide problem. For example, gingival inflammation and heavy calculus deposits were found in 95% of research colony dogs, 2 years old or older, in a study conducted more than 40 years ago. 6 Another early study reported moderate to severe periodontal disease in 75% of necropsied pet dogs that were between 4 and 8 years of age. 7 Recent survey studies of dogs and cats living in several different areas of the world consistently report prevalence rates of periodontal disease of 60% to 80%. 1.8.9. and 10.
Periodontal disease is strongly associated with increasing age in both dogs and cats. In dogs, increasing age is positively correlated with several measures of periodontal disease severity, including the intensity of gingival inflammation, the quantity of calculus that is deposited, and the degree of bone resorption and tooth mobility. 11 Similarly, a study with cats found evidence of periodontal disease in 60% of cats older than 3 years, and another found that 40% of cats older than 7 years of age had advanced periodontitis. 12. and 13. Feline odontoclastic resorptive lesions (FORLs) are also a commonly diagnosed dental disorder of cats as they age. A study of 145 adult cats found evidence of FORLs in 48% of the animals studied. 14 Other groups have found FORL incidence values between 23% and 67%. 15 The progressive nature of periodontal disease and the likelihood that supragingival changes may go unnoticed by owners until there is significant damage to the periodontium may explain the increased incidence in older animals.
Mouth size is a significant risk factor for periodontal disease in dogs. Although all dogs can be affected, the small and toy breeds are more likely to develop periodontitis at an early age and tend to show more severe disease when compared with large breed dogs. 3. and 11. The reduced jaw size and resultant crowding of teeth of small dogs may be predisposing factors to this prevalence. In addition, toy-breed dogs are more likely to have malocclusion problems, which may facilitate the deposition of subgingival plaque that is more difficult to remove either via chewing or through homecare by the owner. As periodontal disease progresses, there is destruction and loss of alveolar bone along the roots of teeth. Because small dogs have a lower ratio of the size of the mandible (lower jaw) to the volume of teeth when compared with large-breed dogs, the loss of bone from the jaw has a greater impact. 16 Alveolar bone loss in small dogs can destabilize the jaw, leading to weakness and eventually to increased risk of fracture. 17
Periodontal disease is the most prevalent type of oral disorder seen by veterinarians. Survey studies of dogs and cats consistently report prevalence rates of periodontal disease of 60% to 80%. Risk increases as pets age, and small and toy breeds of dogs tend to be more severely affected than larger breeds. In cats, feline odontoclastic resorptive lesions are also a commonly diagnosed dental disorder.
Oral Malodor
Oral malodor (halitosis) is commonly reported in dogs and cats and is perceived by many owners to be a significant problem. 18 Moreover, malodor is considered to be a precursor or manifestation of more serious dental disease and is often the first clinical sign that owners report to their veterinarians. As in humans, halitosis in dogs and cats can be caused by oral or nonoral factors. Nonoral etiologies include gastrointestinal, lung, and systemic disease. In the majority of cases in dogs and cats, the predominant source of halitosis is within the oral cavity. Microbial metabolism of protein-containing substances such as food debris, exfoliated epithelium, saliva, and blood result in the production of volatile sulfur compounds (VSCs). 19. and 20. These compounds, particularly methyl mercaptan, dimethyl sulfide, and hydrogen sulfide, produce breath malodor when exhaled. 20 They may also have detrimental effects on the structural integrity of epithelial tissue in the mouth, further contributing to the pathogenesis and progression of periodontal disease. 21
In addition to the microbial flora in the mouth, two other factors that influence the production of malodor in human subjects are the pH and glucose concentration of the saliva. Specifically, saliva with a low pH and a relatively high glucose concentration suppresses odor formation, while the production of saliva with an alkaline pH and low glucose concentration is associated with increased production of odor. 22 Although not reported in dogs and cats, it is presumed that these factors influence breath odor in these species in a similar manner.
Breath malodor is consistently associated with gingivitis and periodontitis. Two independent studies with dogs demonstrated significant correlations between the production of VSCs in the mouth and the amount of plaque and calculus accumulation on the tooth surface. 20. and 23. While the earlier study reported that degree of halitosis was a consistent predictor of the severity of gingivitis, the more recent study did not find a linear relationship with inflammation severity. Another study found that dogs with a high degree of oral malodor were more likely to have moderate to severe periodontal disease when compared with dogs having less malodor. 24 This association is further demonstrated by evidence that veterinary periodontal therapy causes a significant reduction in previously established halitosis in dogs. 25 One explanation for this is that chronic inflammation and tissue damage provides increased protein substrate for microorganisms in the mouth, enhancing the production of VSCs. The heavier plaque that occurs with dental disease may also provide a favorable anaerobic environment and additional substrate for the formation of VSCs. 26
Oral malodor (halitosis) is commonly reported in dogs and cats and is perceived by many owners to be a significant problem. Bad breath is also a sign of more serious dental disease and is often the first problem that owners report to their veterinarians.
Gingivitis and Periodontal Disease
Gingivitis is a nonspecific term referring to inflammation of the gingivae (gums). It is considered to be the reversible stage of periodontal disease and can be prevented by regular plaque removal and control. When a clean tooth surface is exposed to saliva and gingival fluids, a pellicle, composed of a thin layer of proteins and glycoproteins, forms within minutes. Oral debris and plaque-forming bacteria, which are part of the normal oral flora, rapidly adhere to the pellicle and begin to proliferate. Within 24 hours, a smooth layer of plaque covers the entire tooth surface. Newly formed plaque is a soft, gelatinous material composed of bacteria and their metabolic byproducts, oral debris, and salivary components. It is generally thickest along the gingival margin because frictional cleaning associated with normal mastication is much less in this area when compared with the apex of the tooth.
Left undisturbed, aerobic and facultative anaerobic bacteria proliferate as the plaque thickens and matures. As bacterial populations increase, the pellicle becomes more firmly attached to the surface of the tooth and bacterial colonies begin to stratify within the plaque layers. The outer surface of the plaque will contain primarily aerobic, gram-positive species, while the interior surface supports anaerobic populations of bacteria. 27 Species of bacteria that tend to proliferate in dental plaque are classified as “periodontopathogens” because their proliferation and metabolic products contribute to progression of periodontal disease. In dogs, the most commonly isolated periodontopathogens are three species of the black-pigmented anaerobic genus Porphyromonas: P. gluae, P. salivosa, and P. denticans. 28
Over time, salivary minerals, in particular calcium carbonate and calcium phosphate, are deposited within the plaque, producing calculus. Calculus is a hard deposit that provides a rough and porous surface, promoting accumulation of more plaque and also contributing to tissue irritation as it extends into the gingival sulcus.
Gingivitis develops when contact between the plaque bacteria and gingival tissues leads to tissue damage and an inflammatory response. Initially, only supragingival plaque, which is found on the visible portion of the teeth along the gingival margin, is present. As dental plaque matures, it begins to spread under the gingivae. This form of plaque, called subgingival plaque, forms within the gingival sulcus. Left untreated, the gingival sulcus enlarges into a periodontal pocket and the area provides an oxygen-depleted environment that allows proliferation of gram-negative, anaerobic bacteria. Periodontal disease becomes established when the periodontal ligament is exposed to plaque, bacteria, and bacterial byproducts.
Periodontal disease is a plaque-induced, progressive inflammatory disease affecting the gingiva, periodontal ligaments (connective tissue between the tooth root and socket), and alveolar bone. The presence and proliferation of certain species of anaerobic bacteria and the inflammatory responses of the host contribute to the progressive destruction of the periodontium. 1 As the supporting connective tissues and adjacent bone are weakened, teeth become loose and may be lost. Periodontal disease itself causes discomfort and pain and, if left untreated, can lead to bacteremia. For example, a study of 39 dogs with periodontal disease found that 15% of the dogs had bacteremia upon presentation. 29 This increased to 67% after veterinary dental manipulation. The transient increase in bacteremia following dental cleaning was a result of the release of bacteria from disturbed periodontal pockets. There is also evidence that dogs with severe periodontal disease have increased blood bacteria levels immediately following mastication of a meal. 30 Cats with periodontitis are similarly susceptible to bacteremia. 31 As stated previously, the bacteremia associated with periodontal disease is a risk factor for kidney disease, bacterial endocarditis, and pulmonary disease, especially in older animals.
Mature plaque is not removed by normal actions of the tongue or by rinsing of the mouth. Rather, removal requires mechanical abrasion from chewing, regular tooth brushing, and if necessary, veterinary dental cleaning. Therefore pets who do not receive regular home care or veterinary prophylaxis will eventually develop gingivitis. In some animals, gingivitis persists without progressing into periodontitis. However, in most, untreated gingivitis eventually progresses to periodontal disease. Clinical signs of gingivitis and periodontal disease include oral malodor, gingival sensitivity and bleeding, tooth loss, and difficulty eating.
Gingivitis (inflammation of the gums) is considered to be the reversible stage of periodontal disease and can be prevented by regular plaque removal and control. Periodontal disease is a plaque-induced, progressive inflammatory disease affecting the gingiva, periodontal ligaments, and alveolar bone. Untreated gingivitis will eventually progress to periodontal disease in most dogs and cats. Clinical signs of gingivitis and periodontal disease include oral malodor, gingival sensitivity and bleeding, tooth loss, and difficulty eating.
Feline Odontoclastic Resorptive Lesions
Although rare in dogs, odontoclastic resorptive lesions (feline tooth resorption) are one of the most common dental problems reported in the domestic cat. 32. and 33. These lesions are also called neck lesions or cervical line lesions, because the dental defect is often found at the neck area of the tooth. They are characterized by odontoclastic resorption of the tooth’s enamel, dentin, or cementum. 34
Mandibular premolars and molars are the most frequently affected teeth, although canine teeth and incisors may also develop these lesions. As with periodontal disease, the incidence of FORLs is strongly associated with increasing age. 35 Although the underlying etiology of odontoclastic lesions in cats is not completely understood, it is clear that these lesions are not dental caries. Several theories have been advanced to explain FORLs. One study suggested that cats infected with feline immunodeficiency virus were more likely to develop FORLs, which supported virus-induced immunosuppression as an underlying cause. 36 However, other more extensive studies have failed to find corroborating evidence for this theory. 37. and 38. Another theory postulates that FORLs develop in response to localized inflammatory responses associated with gingivitis and periodontal disease. 39 For this reason these lesions are often classified as a form of periodontal disease in the cat. Dietary influences such as the food’s acidifying effects, diet texture (dry versus wet), feeding noncommercial foods, and vitamin D content may also play a role in FORLs (see p. 447).
Because resorptive lesions are very painful to the cat, difficulties in eating and refusal to eat are often the first signs reported by owners. Other signs include oral malodor, gingivitis, and excessive salivation. Gingival inflammation and proliferation are commonly observed in cats with dental lesions, but it is not known if this is a result of the resorptive lesion or an underlying cause. The inflammation associated with FORLs may provide a favorable environment for plaque formation and bacterial proliferation, which lead to gingivitis and possible periodontitis. Alternatively, resorptive lesions may develop in response to the localized and chronic inflammation of gingivae that is associated with periodontal disease. It is known that activity of odontoclasts, the cells responsible for tooth demineralization, is stimulated by chronic inflammation and also by an acidic environment. 34. and 40. In addition to dietary influences in oral pH, bacterial populations associated with chronic inflammatory disease in the cat’s mouth may contribute to an acidic microenvironment necessary for the tooth decalcification that occurs with resorptive lesions.
Initial physical evaluation of a cat’s mouth may not reveal damage to the tooth because of the progressive nature of the disorder. Over time, there is eventual loss of the tooth crown and root. Diagnosis usually requires dental examination and radiographs. 41 FORLs are typically categorized into four stages, with treatment and management procedures dependent upon the stage of the disease at the time of diagnosis. 42. and 43. Although dental prophylaxis and application of a fluoride cavity varnish may stop or slow progression of the early stages, extraction of the tooth is usually necessary in more advanced stages of the disease.
Feline odontoclastic resorptive lesions (FORLs) are one of the most common dental problems reported in the domestic cat. Although the underlying cause is not completely understood, risk of developing a FORL increases in older cats. These lesions are painful and lead to difficulty or refusal to eat in affected cats. Other signs include oral malodor, gingivitis, and excessive salivation. Over time, there is eventual loss of the tooth crown and root; extraction of the tooth is usually necessary in more advanced stages of the disease.
ROLE OF DIET IN THE DEVELOPMENT OF DENTAL DISEASE
The most important factor that influences the development of gingivitis and periodontal disease in dogs and cats is the presence and persistence of undisturbed plaque on tooth surfaces. Therefore management and feeding practices that minimize plaque and calculus formation or aid in their removal are important in the prevention of periodontal disease. Factors that are important include the frequency of tooth brushing, the type of diet that is fed, whether or not table scraps or noncommercial foods are fed, and the frequency of access to chew toys, dental chews, and biscuits.
Once plaque has been deposited on the surface of the tooth, it is most efficiently removed mechanically through the abrasion provided by diet, tooth brushing, or chewing on supplemental chew toys or foods. Chemical agents such as rinses, pastes, and sprays can also be helpful but will not replace the necessary abrasion. For example, the antimicrobial agent chlorhexidine digluconate is effective for the reduction of breath malodor, plaque accumulation, and gingivitis in dogs. 44. and 45. However, the effectiveness of chlorhexidine and other antimicrobial agents is greatly enhanced when they are used in conjunction with brushing. The use of a chemical mouthwash or gel alone is not effective in removing the hardened calculus that forms when plaque is allowed to accumulate. For this reason, an approach that provides frequent and consistent mechanical removal of plaque and calculus is always recommended (see pp. 447-449).
Type of Food: Dry versus Wet
Historically, the form of food that is fed has been implicated as a potential risk factor for the development of dental disease in dogs and cats. Early studies reported that dogs fed a soft (wet) diet developed clinical and histological signs of periodontal disease earlier in life than those fed dry foods. 46. and 47. The severity of disease in dogs fed a soft diet was also greater than that observed in dogs fed a dry biscuit diet. In another early study, dogs fed a food that required mastication did not develop gingivitis during the 1-month trial period. 48 In contrast, dogs fed the same diet in a minced, soft form developed gingivitis and had signs associated with developing periodontal disease.
Survey studies have also been used to identify dietary risk factors associated with periodontal disease in dogs. Data from a group of 63 pet dogs in the United States showed that gingivitis and calculus were less common in dogs fed dry dog food as the major portion of their diet, compared with those fed canned food. 49 However, indicators of tooth mobility, tooth loss, and periodontal disease did not differ significantly with the type of diet fed. Another study conducted by the Japanese Small Animal Veterinary Association collected data from more than 2600 dogs. 50 Analysis showed that dental calculus was found in 34% of dogs fed primarily dry food and 42% of dogs fed primarily canned or home-cooked food. Most recently, a study conducted in Poland provided oral examinations to client-owned dogs recruited by a group of private veterinary practices. 51 Data were collected for almost 30,000 dogs. Following an adjustment for a possible confounding age effect, the data showed that dogs fed only wet food were significantly more likely to have dental calculus and periodontal disease than dogs fed only dry food or a mixture of dry and wet food. Results from these studies indicate that feeding canned foods only may increase risk or severity of periodontal disease. However, the form of the food alone does not completely protect against dental disease; dogs can still accumulate plaque and develop gingivitis and periodontal disease when fed a dry diet.
A similar relationship between wet and dry diet and the development of dental disease was reported in an early study with cats. 52 In this study, the gingivae of growing kittens fed a dry cat food remained healthy, showing little inflammation or accumulation of calculus. In contrast, kittens fed a canned food for the same period developed oral malodor, gingivitis, and calculus. Another group reported that cats had greater plaque accumulation when fed a canned diet, compared with plaque accumulation in cats fed a dry commercial food for a period of only 2 weeks. 53 The Polish survey discussed previously also included 9074 cats. 51 Cats fed wet food only were less likely to be free of dental deposits or periodontal disease than were cats fed dry foods or a mixture of dry and wet food. However, similar to dogs, cats fed dry foods were still at risk of developing dental disease. Current indications are that soft foods such as canned commercial diets or home-prepared foods are less effective than hard, dry foods in providing the abrasion needed to remove plaque that normally forms on teeth. However, the mechanical abrasion provided by feeding a normal dry pet food does not effectively prevent the development of gingivitis and periodontal disease, since in most studies a substantial proportion of animals fed dry diets still developed signs associated with progressive dental disease.
The exclusive feeding of a wet (canned) food or a soft home-prepared diet is less effective at providing the needed mechanical abrasion of chewing to help remove dental plaque than feeding a dry food. However, by itself, the mechanical abrasion from consuming a normal dry pet food cannot effectively prevent the development of gingivitis and periodontal disease in dogs and cats.
Opportunities for Chewing
The dental health benefits afforded by feeding dry pet foods are related to having frequent opportunities for chewing and its associated mechanical cleaning action on the surface of teeth. A study of 1350 dogs in North America examined the relationship between the occurrence and severity of calculus and periodontal disease and the type of diet and chew toys that dogs received. 54 There was a significant linear relationship between decreasing calculus score and increasing number of chewing materials. Although less significant, this trend was also observed for gingivitis. When the type of food alone was considered, there was no significant association between feeding a diet made up exclusively of dry pet food and the degree of calculus, gingivitis, or tooth attachment loss. However, in dogs that were fed dry food, access to rawhide chews and other types of chewing materials was significantly associated with a reduced accumulation of calculus and less gingival inflammation and attachment loss. Rawhide chew materials were the most effective in preventing dental disease, followed by various types of hard bones. In this study, feeding regular (nondental) hard biscuits as a supplement to the dry diet did not provide any additional dental benefit. In contrast, dogs that were not exclusively fed dry food obtained little or no dental benefit from additional chew materials. The authors concluded that there was a consistent (if not always significant) trend toward a widespread protective effect of access to supplemental chewing materials in dogs that were fed dry pet food, compared with dogs fed primarily soft food or a mixture of food types.
A smaller study of 67 dogs showed similar results. 55 Providing dogs with rawhide chews as a supplement to their normal dry diet led to significant removal of preexisting supragingival calculus over a period of 3 weeks. Providing cereal biscuits instead of rawhide was somewhat helpful, but less so than providing rawhide chews. Two other studies examined the dental effects of feeding a rawhide chew that was specifically formulated to promote dental hygiene. 56. and 57. In the first study, dogs were fed a dry maintenance dog food and provided with tooth brushing every other day. 56 Half of the dogs also received one rawhide chew per day. Dental health was assessed over a period of 3 weeks. While plaque accumulation and gingivitis occurred in all of the dogs, significantly less gingivitis developed when the rawhide chew was added to the regimen. After the study period, deposits of calculus and stain were greater with tooth brushing only, compared with the degree of calculus and stain when the teeth were brushed and the chew was provided. In the second study, the effects of the chew on dental health were measured in the absence of regular tooth brushing. 57 Similar results were reported. While gingivitis developed in both groups of dogs, the daily provision of chewing materials significantly decreased the severity. A follow-up study by the same group found that the dental hygiene chew maintained a benefit to oral malodor and the degree of plaque and calculus throughout a 21-month period of offering one chew per day. 58 These results indicate that chewing materials are helpful in reducing the degree of gingivitis through direct mechanical cleaning of the tooth surfaces. However, because dogs given the dental chews still showed some level of plaque accumulation and gingivitis, it appears unlikely that this level of reduction can completely prevent the development of calculus, gingivitis, and periodontitis, even when given to dogs that are exclusively fed a dry diet.
In recent years, dental chew toys that provide mechanical abrasion, and which also include agents to reduce the formation of calculus or inhibit the growth of oral bacteria, have been developed. For example, rawhide treats coated with polyphosphate salts may help to reduce the formation of dental calculus by sequestering salivary calcium. 59 Polyphosphates are also used as coatings on dental diets and have shown some efficacy in reducing calculus formation (see below, pp. 445-446). Another approach is to add an antibacterial agent, such as chlorhexidine, to a dental chew. The intended effect is to reduce bacterial populations present in plaque and reduce their contribution to gingivitis and disease progression. Although one study reported a reduction in plaque accumulation in dogs given this type of chew device, another reported no added efficacy when a toy with an added antimicrobial agent was compared to the same toy without the agent. 60. and 61. It is important that both the efficacy and the safety of supplemented dental chew toys be critically evaluated prior to making any recommendations for their use.
The variable results associated with specific types of chewing toys and different effects on mandibular and maxillary teeth suggest that owners should provide a variety of different types of chewing materials to their dogs. It appears that a cumulative effect is afforded by feeding a dry food and providing additional and varied chew toys. Ideally, dogs fed a dry pet food should have at least one and, if possible, two or more opportunities for extended chewing each day. The additive effect of consuming a dry food plus having frequent access to chew toys may surpass a relative “chewing threshold” that affords some level of protective effect not reached when a canned or soft food is fed and little opportunity to chew is provided. It is also possible that dogs fed dry pet foods may by nature or through learning be more frequent or vigorous “chewers.” This theory is supported by data showing that dogs given rawhide chews varied significantly in their level of interest and in the speed with which they chewed and consumed the rawhide. 62 Videotaping chewing episodes allowed the authors to divide dogs into categories of slow and fast chewers. Dogs classified as slow chewers had less dental calculus accumulation at the end of the 12-month test period when compared with fast chewers, indicating that the amount of time a dog spends chewing each day is an important factor.
While providing chewing toys is beneficial for dental health in dogs, this is generally not an approach that can be used for cats. Although individual cats that enjoy chewing on hard bones or rawhide may exist, most pet cats do not engage in frequent or prolonged bouts of chewing. An examination of the cats’ evolutionary history provides a possible explanation for this difference. Unlike dogs, which evolved from a species that hunted large ungulates and spent a great deal of time chewing bones and tough connective tissue, the cat evolved from the small African wild cat ( Felis libyca), which hunts primarily small rodents, such as mice. The wild cat’s prey is rapidly consumed, with minimal chewing, and numerous mice are caught and eaten each day. As a result, our domestic cat ( Felis catus) has neither the dentition nor (it appears) the desire to spend large amounts of time chewing on bones or other types of chew toys.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


