Dental Disease
5.1 Dental anatomy of the domestic rabbit Oryctolagus cuniculus
The domestic rabbit Oryctolagus cuniculus belongs to the order Lagomorpha. A characteristic of lagomorphs is the presence of four upper incisors. There is a second set of small incisors or ‘peg teeth’ situated just behind the large upper primary incisors. All lagomorph teeth are open rooted (elodont) and, in healthy animals, grow continually throughout life. In myomorph rodents, such as rats, mice and hamsters, only the incisors grow continually; the premolars and molars do not. All the teeth of histricomorph rodents (e.g., guinea pigs and chinchillas) grow throughout life but histricomorphs rodents do not have peg teeth. No rodent species have two sets of teeth (simplicidentata).
Rabbits have two sets of teeth (duplicidentata). A deciduous set is present in fetal rabbits and is shed just before or just after birth (Wiggs and Lobprise, 1995). The deciduous set comprises:
The permanent set of teeth erupts during the first 5 weeks of life and comprises:
Rabbits’ teeth have the same structural components as other animals, i.e., dentine, enamel, cementum and pulp. The main body of the tooth is made up of dentine composed of hydroxyapatite crystals similar to those in bone, but much denser. The crystals are embedded in a collagen matrix that is also similar to bone but without the osteocytes, osteoclasts, osteoblasts or blood vessels. Dentine is nourished by a layer of odontoblasts that line its inner surface along the pulp cavity. The odontoblastic layer contains free sensory nerve endings, some of which extend into the dentine through tubules. Species with continually erupting teeth, such as rabbits, have smaller and fewer axons in the dentine than animals with permanent dentition (Byers, 1984). In the rabbit, tubular dentine is formed at the apex of the tooth and becomes thicker as it migrates occlusally. In the premolars at least this dentine is innervated (in contrast to rat incisors) and Bishop (1995) suggests that this is for nociception rather than for sensory control of mastication. Enamel is formed at the apex by a layer of ameloblasts (Bishop, 1995).
The upper incisor teeth have a single deep groove on the labial aspect that runs longitudinally along the length of the tooth. The amount of enamel covering the crowns of the incisors varies with each type of incisor tooth (Hirschfield et al., 1973). The large upper primary incisors have a thick layer of enamel on the labial aspect but none on the lingual side. The lower incisors have enamel on both the labial and lingual aspects. This distribution of enamel permits the formation of sharp cutting edges to the tips of the teeth (see Figure 5.1). The incisors are primarily used for slicing through vegetation, although they can be used for gnawing and biting. Canine teeth are absent. There is a large diastema or space between the incisors and premolars. The premolars and molars are indistinguishable from each other and form a row of grinding cheek teeth.
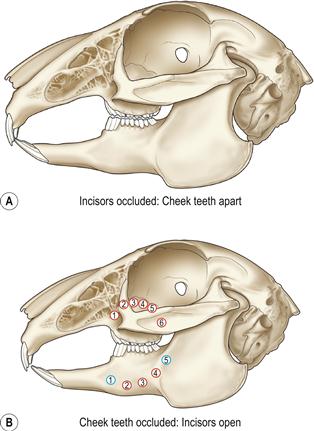
Figure 5.1 Normal incisor occlusion and sites of bone penetration by elongated roots of the cheek teeth.
The incisors are used to slice through vegetation like scissors. The lower incisors can also be moved in a rostrocaudal direction to gnaw through bark or wood. The large upper primary incisors have a thick layer of enamel on the labial aspect but none on the lingual side. This distribution of enamel permits the formation of sharp cutting edges to the tips of the upper primary incisors as the lingual aspect is worn against the lower incisor. During grinding of food between the cheek teeth, chewing movements in rabbits are made in a lateral direction (see Figure 5.9), rather than the rostrocaudal action that takes place in rodents. During mastication, the jaw is moved through an arc that also brings the mandibular incisors into contact with the peg teeth. This action wears the tips of the mandibular incisors to a cutting edge. This figure shows the mandible at the caudal extent of its range of motion, allowing the cheek teeth to be in apposition. At the rostral extent of the mandibles range the incisors are in occlusion and the cheek teeth a little open.
Root elongation occurs as part of the syndrome of ADD that affects pet rabbits. Alterations in the shape and curvature of the teeth tend to follow a characteristic pattern. Elongated roots penetrate the bone and emerge through the periosteum in predictable sites. Periapical abscesses occur at the site of root penetration. The site of the abscess can be helpful in predicting which tooth root is infected. In the figure the penetration sites of elongated roots of the cheek teeth (molariform teeth) are shown. The red circles are roots that curve and penetrate the bone laterally. The blue circles are roots that curve and penetrate the bone medially. The circle contains the number of the tooth so, the fourth molariform tooth, deviating laterally, is  .
.
The mandibular cheek teeth are arranged in a straight line. The maxillary cheek teeth are similarly arranged, except that the intermediate premolars and molars are wider than the first premolar and the last molar, giving the buccal side of the alignment a convex shape. The circumference of the cheek teeth exhibits deep grooves or embrasures, which are deep on the buccal aspect and fit into a corresponding groove in the alveolar wall (see Figures 5.2 and 5.3).
Longitudinally, the root and the crown of the cheek teeth are morphologically indistinct (see Figures 5.2 and 5.3). The term ‘reserve crown’ is more correctly used to describe the root. Wild rabbits and those pet rabbits that eat grass and natural vegetation sometimes have brown staining on the supragingival crowns of the teeth, especially the cheek teeth (see Figures 5.4–5.7).
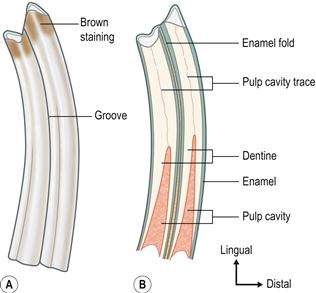
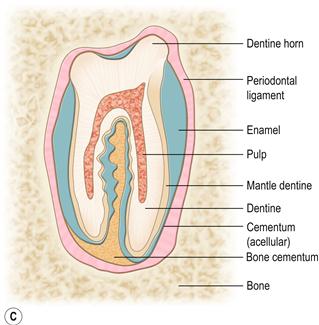
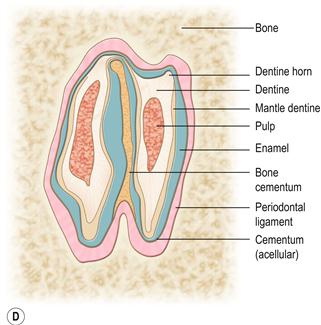
Figure 5.2 Structure of the cheek teeth.(A) Lateral aspect of the second lower right molar (molariform tooth number 4).
The premolars and molars are very similar. Some texts use the term ‘molariform teeth’. Longitudinally, the reserve crown and the clinical crown of the cheek teeth are not morphologically distinct. Wild rabbits and those pet rabbits that eat grass and natural vegetation may have brown staining on the supragingival (clinical) crowns of the teeth (see Figure 7.17).(B) Longitudinal section through the second lower right molar.
It shows the two laminar extensions of the pulp cavity. These extensions converge at the apex of the tooth to form a single pulp chamber that contains a range of differentiated and undifferentiated cells and a nerve supply. Towards the occlusal end, the two pulp chambers taper and close, and the dentine transforms from tubular to thicker atubular tissue in which there are no blood vessels. There is a deep longitudinal fold of enamel in the centre of the tooth, which can be seen as a radiodense line in the cheek teeth on lateral radiographs of the skull.(C) Transverse section through a maxillary (upper) cheek tooth and (D) transverse section through a mandibular (lower) cheek tooth.
The external surface of the cheek teeth is made of enamel that is covered by a layer of acellular cementum into which the fibres of the periodontal ligament are embedded. The opposing ends of the fibres are embedded into alveolar bone anchoring the tooth into the alveolar socket. The circumference of the mandibular cheek teeth exhibits deep grooves that fit into a corresponding groove in the alveolar wall. All the cheek teeth, except the first and last of the upper jaw, possess an enamel fold which runs across the tooth from the vestibular to lingual surface. The fissure in the enamel fold is filled with a type of vascular bone cementum that is contiguous with the avascular cementum surrounding the tooth. On the lower cheek teeth, this enamel fold almost divides the tooth in two.
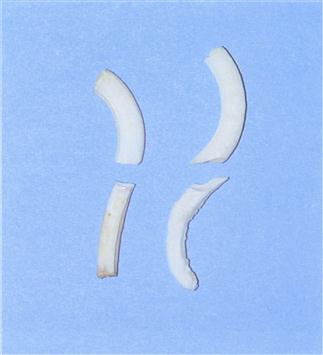
Figure 5.3 Comparison of the structure of healthy and maloccluded cheek teeth.
An upper and lower premolar taken from the skull of a healthy rabbit (left) and a rabbit suffering from molar malocclusion (right). The maloccluded teeth are curved and elongated. The enamel is poor. Curvature of the teeth has altered the direction of the biting force on the teeth. The changes in tooth shape are irreversible and show that it is impossible to restore normal occlusion by corrective dentistry.
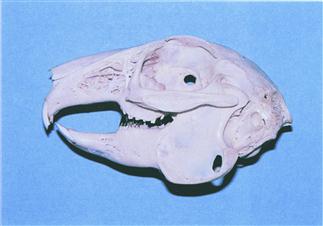
Figure 5.4 Wild rabbit skull: lateral view.
A prepared skull of an adult wild female European rabbit (Oryctolagus cuniculus) that was found dead in Menorca, a limestone Mediterranean island with plenty of sunshine. The skull is well calcified. The teeth show brown staining that often occurs in rabbits that eat grass and other natural vegetation.
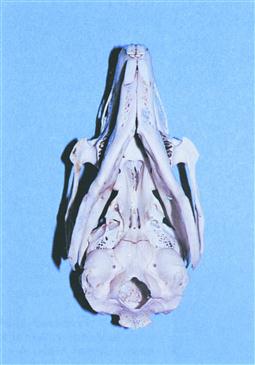
Figure 5.5 Wild rabbit skull: ventrodorsal view.
A ventrodorsal view of the same skull as in Figure 5.4. These views can be used to identify radiographic landmarks illustrated in Figures 5.17, 5.19 and 5.22.
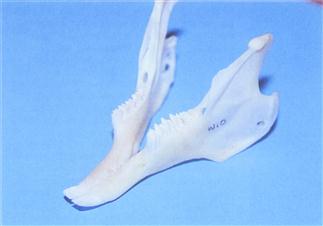
Figure 5.6 Healthy mandible to show points on the cheek teeth.
A prepared mandible from a wild rabbit. Sharp points on the lingual edge of the lower cheek teeth is a normal finding. They do not require removal.
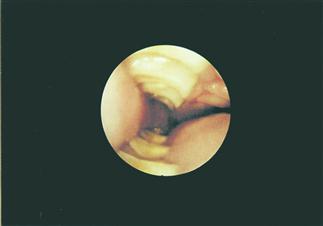
Figure 5.7 Otoscopic view of normal cheek teeth.
The cheek teeth can be examined using an otoscope. Otoscopic examination gives a guide to the condition of the cheek teeth. General anaesthesia is required for a thorough examination. This is a view of the cheek teeth of a conscious healthy 3-year-old female rabbit taken through a rigid endoscope. The normal zigzag pattern of the points on the lingual edge of the occlusal surface on the lower cheek teeth can be seen. Most rabbits tolerate the otoscopic examination of the oral cavity well, unless there is a painful lesion within the mouth.
Examination of transverse sections across the long axis of the cheek teeth shows the pulp cavity in the two laminar extensions that converge at the apex of the tooth to form a single pulp chamber containing a range of differentiated and undifferentiated cells and a nerve supply. At the occlusal end, the two pulp chambers taper and close, and the dentine transforms from tubular to thicker atubular tissue in which there are no blood vessels (Bishop, 1995). There is a deep longitudinal fold of enamel in the centre of the molars and premolars (see Figures 5.2 and 5.8). This fold can be seen as a radiodense line running longitudinally in the cheek teeth on lateral radiographs of the skull (see Figure 5.14). The circumference of the tooth is made up of enamel covered by a layer of acellular cementum in which the fibres of the periodontal ligament are embedded. The opposing ends of the fibres are embedded into alveolar bone, anchoring the tooth into the alveolar socket. Like the incisors, the cheek teeth are kept in shape by a continual process of growth and attrition. The soft cementum and harder dentine of the occlusal surface are worn away before the enamel, which survives as sharp edge, both at the circumference and across the centre of the tooth. This gives the molars and premolars an effective shredding surface (Michaeli et al., 1980). The enamel fold forms a ridge across the centre of the occlusal surface that interlocks with the interdental space between the two occluding teeth (see Figure 5.2). This occlusal pattern results in a rostrocaudal succession of transverse ridges and valleys along the surface of the cheek teeth. Upper ridges are reciprocal with lower valleys and vice versa. The pattern gives a characteristic zigzag pattern on lateral radiographs (see Figure 5.14).
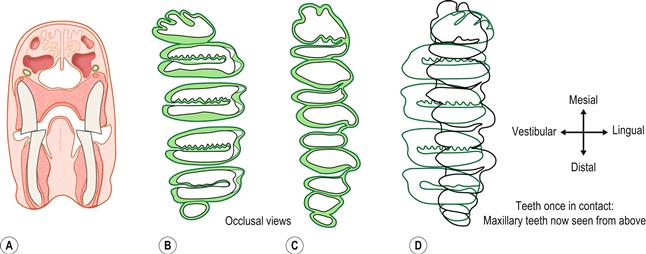
Figure 5.8 Occlusion of the cheek teeth.(A) Transverse section through the head of a wild rabbit shows the anatomical relationship of the upper and lower cheek teeth.
This was drawn from a decalcified head sectioned at the level of the interdental space between the first and second lower premolars. The upper second premolar has been sectioned across the tooth. The head was taken from a mature adult wild rabbit with no evidence of dental disease. It can be seen that the jaws are isognathic; i.e., the rows of teeth in the lower jaw are closer together than those in the upper jaw. This only allows the cheek teeth on one side of the mouth to be in occlusion at any time.(B) Occlusal surfaces of the (left) maxillary cheek teeth; (C) occlusal surfaces of the (left) mandibular cheek teeth; and (D) superimposition of the outline of the maxillary cheek teeth onto the mandibular cheek teeth to show their occlusal relationship.
In (B) and (C) the illustrations were drawn from the teeth of a wild rabbit. In Nomina Anatomica Veterinarium, the five surfaces of the teeth are named occlusal, mesial and distal, lingual and vestibular. Vestibular replaces buccal and labial. The vestibular surface is so named because this surface faces vestibulum oris.
There are six molariform cheek teeth in the upper jaw and five in the lower jaw. The mandibular cheek teeth are arranged in a straight line. The maxillary cheek teeth are similarly arranged, except that the intermediate premolars and molars are wider than the first premolar and the last molar, giving the buccal side of the alignment a convex shape (B). The first and last teeth of the lower jaw are larger than the opposing tooth of the upper jaw. All the teeth, except the first and last of the upper jaw, possess an enamel fold which runs across the middle of the tooth from the vestibular to lingual surface. Because of the enamel fold, each tooth appears to consist of two parts. If viewed in this manner there are 10 molariform ‘tooth parts’ in each jaw, each one occluding with an opposite part. The teeth are arranged in staggered manner so that the enamel fold of one tooth corresponds to the mesial or distal surface of the opposing tooth. This relationship of the occlusal surfaces is illustrated in (D).
This arrangement forms an effective grinding surface that is used to sever tough fibrous plant material. The grinding effect of the teeth is enhanced by the enamel that forms the circumference of the tooth and the enamel fold in the centre of the tooth. The central ridge of enamel in the upper molariform teeth is convoluted and has the appearance of a serrated edge. This serrated edge corresponds to the enamel ridges of the distal part of one tooth and the mesial part of the next. The lingual aspect of the occlusal surface of the cheek teeth forms two raised ‘horns’. These horns are greater on the lower cheek teeth than on the upper cheek teeth.
The grinding occlusal surface of the teeth is maintained by attrition. Attrition is tooth wear caused by rubbing or grinding against the opposing tooth in the absence of food material. Dental wear is affected by the abrasive nature of the diet. The effect of attrition and abrasion can be seen by examining the occlusal surfaces. Dental wear caused by attrition can be seen as polished facets on the occlusal surfaces of the teeth. Abrasion is characterized by scratched surfaces on the tooth. In healthy rabbits, polished facets can be seen on the occlusal surfaces. During mastication, the cheek teeth grind food by moving the lower jaw laterally. The mandibular movements are guided by the ridges and valleys in the teeth and are unidirectional. The occlusal configuration and the forces that occur during the chewing move the jaw back to the midline during contact with food (see Figure 5.9).
The occlusal pattern of the cheek teeth is anisognathic; i.e., the mandible is narrow relative to the maxilla (see Figure 5.9). When the jaws are closed, the mandibular cheek teeth lie inside those of the upper jaw with the buccal edge of the lower cheek teeth just touching the palatal edge of the upper cheek teeth.
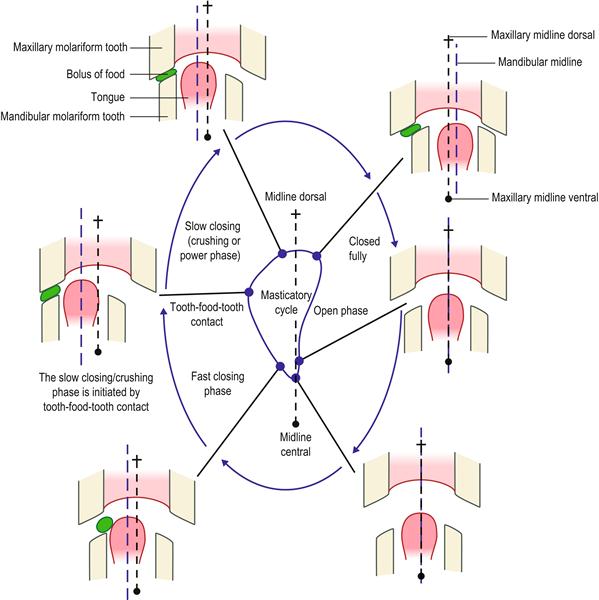
Figure 5.9 Movement of jaw during grinding of food between cheek teeth (type II masticatory cycle).
The jaw movement trajectory of rabbits has been traced using magnetic sensors placed at various sites on the head (Schwartz et al., 1989; Yamada and Yamamura, 1996). Masticatory actions can be divided into three sequences or ‘types’. These are described in Section 5.2. Type I is the preparatory masticatory sequence in which the incisors cut through food to reduce it to manageable pieces that are transported by the tongue to the posterior teeth for reduction. Type II is the sequence of jaw movements that take place during grinding of food between the cheek teeth. Type III is the masticatory sequence that takes place before the bolus of food is swallowed. This is a more complex cycle that has five phases.
This figure summarizes the movement of the mandible during the type II chewing cycle. Jaw movement follows a unidirectional crescent-shaped movement. Either side of the mouth may be used to chew food, but only one side can be used at a time. The mandible is always moving towards the midline when food is crushed between the teeth. The force with which the food is crushed is directly proportional to the hardness of the food. The crushing phase is initiated by contact with food between the teeth. Sensory input to the feedback mechanism is transmitted through the trigeminal nerve from pressoreceptors in the periodontal ligament and muscle spindles in the muscles of mastication.
5.2 Mastication
Mastication has been studied extensively in rabbits. The masticatory sequence can be divided into three sequences or ‘types’. During each type of masticatory cycle there are several phases of jaw movement.
Type I is the preparatory masticatory sequence that has two phases: a jaw opening phase and jaw closing phase. During this sequence, the incisors cut through food to reduce it to manageable pieces that are transported by the tongue to the posterior teeth for reduction. Jaw movement is predominantly in the sagittal plane with small lateral excursions away from the midline.
Type II is the reduction sequence of masticatory movements that occurs when food is ground down between the cheek teeth. During type II mastication, chewing can only take place on one side of the mouth at a time. Lateral excursion is wide and the jaw follows a unidirectional crescent-shaped movement throughout the chewing cycle (see Figure 5.10). There are three phases in the type II masticatory sequence: a jaw opening phase; a fast closing phase; and a slow closing phase during which food is crushed between the teeth (see Figure 5.10). Although the upper and lower cheek teeth often do not come in contact during mastication (Schwartz et al., 1989), the mandibular movements are guided by the ridges and valleys in the teeth (Langenbach et al., 1991). The occlusal configuration and the medially directed forces that occur during chewing drive the jaw back to the midline during contact with food. The basic chewing rhythm is not affected by the food texture (Yamada and Yamamura, 1996) although the force that is applied by the teeth during crushing increases in proportion to the hardness of the food. Sensory input to the feedback mechanism comes from pressoreceptors in the periodontal ligament and muscle spindles in the muscles of mastication.
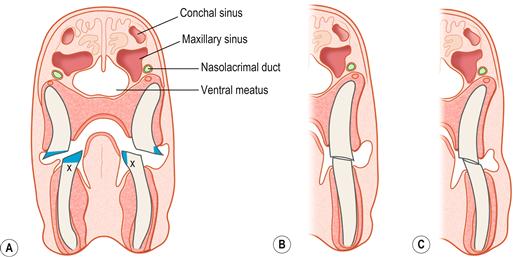
Figure 5.10 Cheek tooth malocclusion and root elongation (the position of the conchal and maxillary sinuses is also illustrated).(A) Trimming cheek teeth.
This illustration was drawn from a transverse section of a decalcified head of a rabbit that was euthanized because it had developed molar malocclusion. Cheek tooth malocclusion and root elongation are part of the syndrome of ADD that affects pet rabbits. The whole structure, shape and position of the teeth alter. The teeth become long and curved. Sharp spurs develop on the mandibular molars that impinge on the tongue and interfere with chewing, licking and grooming. In advanced cases, these spurs can lacerate the tongue and cause substantial tissue damage (see Figures 5.18 and 5.20). The effect of trimming these spurs is illustrated here. Although, logically, it would seem that reducing the crowns to gum level should increase the time it takes for sharp spurs to regrow, this is not the case. Here, the letter X marks the part of the tooth that will grow into in a spur. It can be seen from the diagram that trimming off the spur, and leaving an occlusal surface on the teeth has the same effect on X as removing the crown completely. Grinding the teeth down to gum level is counterproductive for several reasons:
In (A) the position of the conchal sinus and maxillary recess is also shown. The anatomical relationship of the paranasal sinuses with the nasal passages is also illustrated in Figure 11.2A. The conchal sinus and maxillary recess have a common entrance in the caudal nasal cavity. The rostral end of both cavities is blind ending, which prevents drainage of exudate from the sinuses if they become infected (see Figure 11.2).(B, C) How alterations in the shape of the teeth affect the occlusal forces placed on them during chewing.
In order to grind food, the lower jaw is moved laterally and only one side of the mouth is used at any one time (see Figure 5.9). In (B, C) the mandible has been moved laterally to bring the teeth into occlusion; (B) shows the occlusion of the cheek teeth during mastication in a healthy rabbit; (C) shows the occlusion of the cheek teeth during mastication in a rabbit with cheek tooth malocclusion. Increased force is applied to the lingual edge of the lower cheek teeth and to the buccal edge of the upper cheek teeth. Where there are spurs in these positions, it is painful for the rabbit to make lateral chewing motions (e.g., when eating grass or hay). Vertical tooth movement (for example, used when eating pellets) may be less affected. A reluctance to eat fresh food/hay may be an early sign of dental disease. These illustrations are drawn from actual post-mortem specimens.
The third (type III) masticatory sequence is the pre-swallowing series of jaw movements that includes an additional two opening phases during which the bolus of food is swallowed.
5.3 Factors that affect tooth shape in rabbits
In healthy rabbits, the teeth are kept in shape by continual growth and attrition. Attrition is the occlusal wear of tooth against tooth. The softer dentine is worn away more quickly than the hard enamel that remains as sharp-edged ridges that cut against each other like scissors (see Figures 5.2, 5.8). Dental wear is affected by contact with food and the abrasive nature of the diet.
Rabbits can be seen grinding their teeth when they are at rest. There is evidence of dental wear from bruxism (tooth grinding) on the non-functional deciduous teeth of neonatal rabbits (Wiggs and Lobprise, 1995). Early zoologists believed that rabbits were animals that chewed cud (Shadle, 1936; Taylor, 1940). It is not clear whether this belief arose from observations of chewing behaviour or from the presence of caecotrophs in the stomach.
The rate of growth and attrition is variable between individuals and is also influenced by pregnancy, age and diet (Lowe, 1998; Ness, 1956; Shadle, 1936). The constant process of growth and attrition demands a supply of calcium and other minerals and nutrients for the formation of dentine and enamel. The periodontal space contains a capillary network that provides ameloblasts with nutrients (Okada et al., 1990). The upper incisors do not grow as quickly as the lower incisors. Hamidur Rahman et al. (1983) recorded a rate of approximately 12.7 cm (5 in.) per year in the upper incisors and 20.3 cm (8 in.) per year in lower ones. These findings are similar to those of Shadle (1936), who recorded a growth rate of approximately 2 mm per week for the upper incisors and 2.4 mm per week for lower ones.
The speed at which crowns grow is not only determined by the rate of attrition but also the rate of eruption. Taking teeth out of occlusion and preventing attrition hastens the rate of eruption. Incisors were found to erupt at a rate of 280 μm/day (0.28 mm/day) in a study by Ness (1956) in which amalgam markers were placed in the labial surfaces of the teeth. Shortening the teeth and taking them out of occlusion increased the eruption rate to 700 μm/day (0.7 mm/day).
Dental wear is affected by the abrasive nature of the diet. Dental abrasion is caused by silicate phytoliths that are present in the skeleton of grasses. Cellulose and lignin are also abrasive. Abrasion results in microscratches on the occlusal surface of the tooth, whereas dental wear by attrition results in polished facets (Hillson, 1986). The diet of wild rabbits is naturally abrasive and requires grinding down to small particles before it can be swallowed and digested. By nature, rabbits are destructive creatures that strip bark off trees and chew through tree roots in addition to grazing and browsing. The abrasive nature of the diet is an important factor in the maintenance of normal occlusion. Wolf et al. (1993) studied the effect of diet on incisor growth and attrition. Groups of rabbits were fed on pelleted rations, cereal mixtures, green forages with or without hay and gnawing blocks. They found the biggest difference between the rate of tooth eruption and rate of attrition occurred in rabbits fed on a complete pelleted diet and the lowest difference occurred in rabbits fed on a diet of green forage. They concluded that the duration of feed intake might be more important than the hardness of feed as a determining factor in the rate of growth and attrition.
5.4 Dental disorders of pet rabbits
Dental disorders have been recognized as a cause of disease in rabbits for many years. Excessive salivation in association with abnormal teeth was described as ‘slobbers’ by Pollock (1951) and this term is still used in many texts. Until recently, dental disorders were considered to be congenital but it has now become evident that other factors are involved, although congenital malocclusion does occur. Rabbits’ teeth are precisely arranged and aligned to wear against each other to maintain their shape and occlusion (see Figure 5.8). Any factor that alters the relative position of a tooth, even by a fraction, can result in the development of malocclusion and the formation of elongated crowns.
There is a progressive syndrome of acquired dental disease (ADD) and malocclusion in rabbits characterized by elongation of the roots of the teeth. Although this syndrome is very common in pet rabbits, it is scarcely reported in laboratory or commercial rabbits. Weisbroth and Ehrman (1967) recognized ectopic tooth roots could penetrate the bones of the skull and result in abscessation. They believed the condition to be inherited and included no details of the diet and husbandry in their description of affected rabbits. A request was made for ‘reasons, suggestions or comments by interested persons’ on the development, pathology and inheritance of malocclusion in rabbits. There is a subsequent individual case report (Ireson, 1968) in which elongation of the maxillary tooth roots is noted.
Acquired malocclusion was recognized and described by Zeman and Fielder (1969), who wrote ‘the nature of premolar and molar malocclusion seems to defy at this time any positive determination of the cause’. Speculation and debate on the aetiology of ADD and malocclusion in rabbits has continued ever since (see Box 5.2 below). Rabbit breeders have attributed ‘slobbers’ to a faulty diet, sudden changes in food, lack of sunlight or cold and damp hutches (Pollock, 1951). Some authors believe that cheek tooth malocclusion is a result of abnormal wear caused by incisor malocclusion (Jenkins, 1997) or that disease of the temporomandibular joint may be responsible (Wiggs and Lobprise, 1995). Jenkins (1997) suggests ageing as a cause of cheek teeth malocclusion and Brown (1992) cites inflammation of the molar roots as a cause of primary molar malocclusion leading to secondary incisor malocclusion. Westerhof and Lumeij (1987) suggested that lack of hard food might be a predisposing factor for cheek teeth problems. Harcourt-Brown (1995) described a number of clinical conditions in rabbits caused by dental problems. The skulls of affected rabbits had a visual osteodystrophic appearance, suggestive of metabolic bone disease.
5.5 Acquired dental disease in pet rabbits
There is a syndrome of ADD in pet rabbits that is a progressive condition and can be staged (Harcourt-Brown, 1997) (see Box 5.1). The disease is characterized by deterioration in tooth quality, acquired malocclusion and elongation of the tooth roots. Periapical abscesses frequently occur. The progression of ADD is described in Box 5.1 and illustrated in Figures 5.3–5.7, 5.11, 5.12, 5.15, 5.16, 5.18, 5.20 and 5.22. The exact aetiopathogenesis of this syndrome is not clear and several factors are probably involved, including metabolic bone disease, dietary texture and genetic predisposition. Experimentally, poor bone quality will exacerbate the effect of abnormal loads on rabbits’ teeth, making them more susceptible to displacement and distortion. Ashcraft et al. (1992) found that orthodontic tooth movement was three to four times greater in rabbits with corticosteroid-induced osteoporosis than in the untreated control group. In rats, hypocalcaemia leads to enhanced alveolar bone resorption and an increased susceptibility to root resorption in response to a moderate orthodontic force (Engstrom et al., 1988).
ADD typically occurs in pet rabbits that are housed indoors, bedded on hay and fed on mixed cereal rations, with or without occasional vegetables and sporadic access to the lawn. Wild rabbits and pet rabbits that live outside all year round with unrestricted access to grazing and browsing do not develop this syndrome, whatever their breed. Housed pet rabbits that eat well and consume a diet rich in grass, hay and vegetables are less likely to develop ADD than those rabbits that select certain ingredients from mixed rations and consume a diet that is low in indigestible fibre.
5.5.1 Causes of acquired dental disease in pet rabbits
Acquired dental disease in rabbits is usually attributed to lack of dental wear. Many handbooks and leaflets suggest that twigs and branches should be given to pet rabbits for them to gnaw on and wear their teeth down. There is a variety of artificial chews and blocks available from pet shops for this purpose, but very often these are only gnawed using the incisors, and therefore fail to wear the cheek teeth down. Lack of dental exercise and uneven wear has been suggested as a cause of enamel spurs on the cheek teeth (Crossley, 1995a). Although a fibrous diet and long periods of chewing keep tooth crowns short, the lack of an abrasive diet and dental exercise cannot be the only cause of ADD in rabbits. The progressive syndrome of ADD has only been described in pet rabbits and has not been described in laboratory or commercial rabbits. Instead, congenital defects that affect occlusion have been reported (Lindsey and Fox, 1994; Okerman, 1988). Laboratory and commercial rabbits are usually fed on a complete pelleted ration. Additional hay is not always provided and the diet of these animals is not always abrasive. Laboratory rabbits have been maintained for one year on a complete liquid diet without mention of dental problems (Latour et al., 1998). A complete purified diet based on agar gel was fed to a group of laboratory rabbits for up to 2 years with no gross or histopathological lesions attributable to nutritional disease being observed during autopsy (Hunt and Harrington, 1974).
5.5.1.1 Metabolic bone disease as a cause of dental disease in rabbits
Evidence for metabolic bone disease as a cause of ADD in pet rabbits is given in Box 5.2. Metabolic bone disease includes (but is not limited to) a range of inter-related conditions such as rickets, osteoporosis, nutritional osteodystrophy and nutritional secondary hyperparathyroidism. Metabolic bone disease is defined by Fowler (1986) as ‘a disease caused by dietary and husbandry mismanagement characterised by metabolic defects affecting the morphology and functioning of bones. The clinical, radiographic and pathological manifestations vary with the age of the animal, species of the animal, degree of deficiency, duration of the deficiency and presence of concurrent diseases’. Metabolic bone disease occurs in many species whose natural characteristics and lifestyle involve exposure to sunlight. Problems develop with calcium metabolism when animals are housed indoors and fed on foods that do not match their natural diet in the wild. The effects of metabolic bone disease vary with species and can be manifested in a variety of ways. For example, shell deformities in growing tortoises, pathological fractures of iguanas or hand-reared birds, dental malocclusion in monkeys (Fowler, 1986) or reduced bone density in ponies (El Shorafa et al., 1979). Nutritional osteodystrophy has been described as a cause of acquired dental malocclusion in species such as sheep and monkeys (Duckworth et al., 1961; Fowler, 1986; McRoberts et al., 1965). Demineralization of supporting alveolar bone results in tooth movement and the development of malocclusion. In calcium-deficient rats, demineralization of alveolar bone occurs before changes are seen in other bones, such as the femur (Abe et al., 1989).
Mineral deposition in the teeth is affected by the availability of calcium, phosphate and vitamin D (Guyton, 1991). The fast rate of growth of rabbits’ teeth means that there is a high demand for calcium for the continual formation of dentine and enamel. In other species with continually erupting teeth, such as rats, a diet deficient in calcium and vitamin D results in enamel hypoplasia after only 4 weeks (Engstrom and Noren, 1986) and hypomineralized enamel develops on the incisors of juvenile rats weaned onto a calcium-deficient diet (Lozupone and Favia, 1989). Growth and reproduction increase susceptibility to calcium and/or vitamin D deficiency. Young rabbits have a higher demand for calcium during the growing period and dietary imbalances can have lifelong effects on the shape and structure of the skeleton, including the bones of the skull. At the other end of the scale, ageing may also affect tooth and bone quality. Pregnancy and lactation have a considerable influence on bone density in rabbits (Julius, 1997).
Poor bone calcification in pet rabbits was reported by Wood (1978), who recommended that treatment of fractures should include vitamin D and calcium therapy. A feature of ADD in rabbits is poor calcification of the bones of the skull, especially the alveolar bone that surrounds and supports the teeth. Loss of alveolar bone at the apex of the tooth allows the continually growing roots to elongate and eventually penetrate the periosteum (see Figures 5.11, 5.12 and 5.15). Loss of surrounding alveolar bone that supports the teeth results in distortion and loosening of the teeth, which causes uneven wear on the occlusal surfaces. The relative position of the teeth changes, which also alters occlusion. Enamel is lost both from the circumference and the centre of the cheek teeth so the shredding occlusal surface cannot be maintained. The whole shape, structure and position of all the teeth is affected by the syndrome of ADD (see Figures 5.3 and 5.13–5.16).
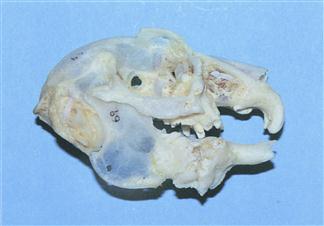
Figure 5.11 Prepared skull of a rabbit with advanced dental disease: lateral view.
This is a skull of a 4-year-old Netherland dwarf female rabbit that was presented for euthanasia. She had spent her entire life confined to a hutch and was fed on ad lib ‘rabbit food’ consisting of a mixture of maize, peas, wheat, oats, pellets and extrusions. Hay was available although she didn’t eat it. No vegetables were offered because the owner believed they would cause ‘diarrhoea’. She was suffering from dacryocystitis and inappetence. The skull shows generalized osteopenia. The roots of all the teeth are elongated and penetrating periosteal bone. Calcification around the roots has resulted in large bony reactions that are effectively welding the teeth into the skull. There is a large bony reaction in the orbit around the site of the lacrimal sac and nasolacrimal duct. The crowns of most of the teeth have broken off.
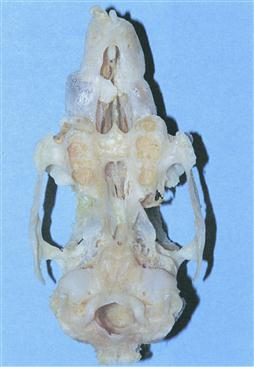
Figure 5.12 Prepared skull of a rabbit with advanced dental disease: ventrodorsal view.
A ventrodorsal view of a prepared skull of a 4-year-old male dwarf lop rabbit. He was kept in a hutch for most of the year but was placed in a run in the garden on fine days during the summer months. The rabbit was always a finicky eater; he would (or could) not eat hay or hard vegetables. He lived on bread and selected ingredients from mixed rabbit food. He would eat watercress and the occasional dandelion leaf. During his life, the rabbit had suffered from a range of clinical conditions related to his teeth: epiphora, dacrocystitis, molar malocclusion and incisor malocclusion. The progression of dental disease is summarized in Box 5.1. The rabbit was euthanized because he became dyspnoeic. The skull shows osteopenic bone and dilation of the maxillae. Abscesses have developed at the roots of the primary incisors, which are occluding the nasal passages. The crowns of the cheek teeth have disintegrated.
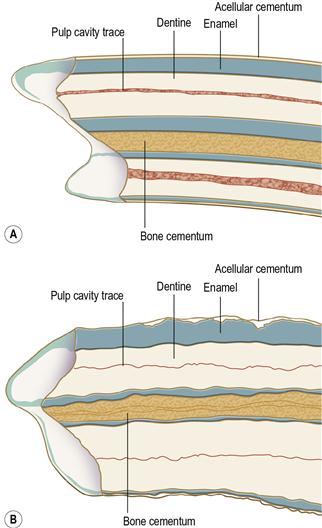
Figure 5.13 Comparison of the structure of healthy (A) and diseased (B) mandibular cheek teeth.
(A) was traced from a longitudinal section through a healthy mandibular molar from an adult pet rabbit unaffected by dental disease; (B) was traced from a longitudinal section through a first mandibular molar from an adult rabbit suffering from ADD. A comparison of the two illustrations shows that there are changes to the enamel, dentine and cementum throughout the diseased tooth. These changes have reduced the hardness of the tooth and altered the pattern of dental wear on the occlusal surface. These alterations in the strength and structure of the cheek teeth of rabbits with ADD leads to uneven wear and the development of ‘step mouth’ (see Figure 5.22B).
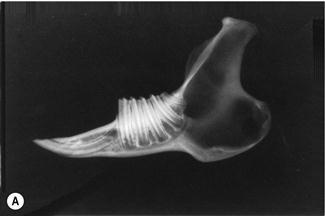
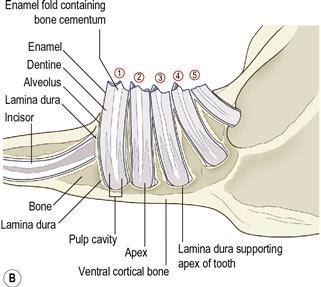
Figure 5.14 Radiographic anatomy of teeth and surrounding jaw.
(A) Radiograph and (B) diagram of the lateral view of a prepared hemi-mandible taken from an adult rabbit unaffected by dental disease. The radiographic anatomy of the tooth and surrounding structures can be seen.
Each alveolus (tooth socket) is bordered by the lamina dura, which is a line of radiodense bone that contains highly calcified cementing substance into which the periodontal ligament binds. The lamina dura and the wall of the tooth are separated by a space that contains the periodontal ligament. Each tooth has a central dense line formed by the enamel fold and the bone cementum that lies within it. Towards the apex of the tooth, on either side of the enamel fold, two tapering longitudinal radiolucent areas are formed by the pulp cavity. These areas become wider towards the apex, where they join to form a single radiolucent area that consists of blood vessels, nerves and germinal tissue. This area is also bordered by lamina dura. The ventral aspect of the normal mandible is a smooth bar of cortical bone.
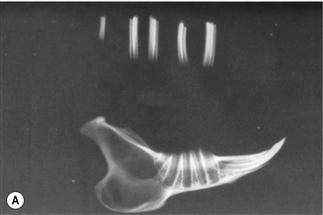
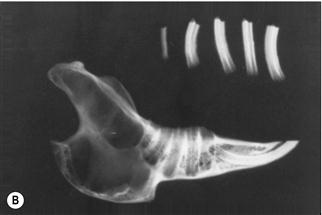
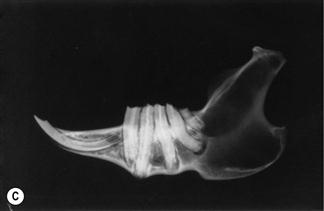
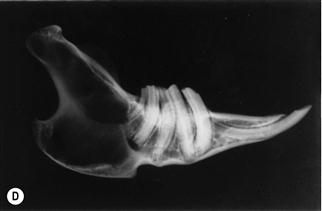
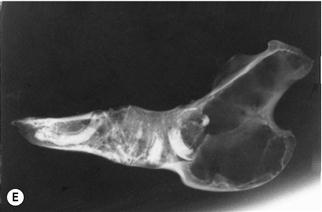
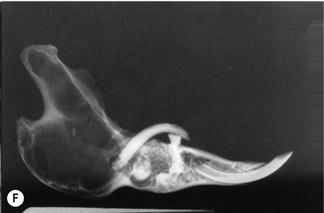
Figure 5.15 Progression of acquired dental disease.
The changes that take place within the teeth and surrounding jaw of rabbits with ADD can be monitored radiographically: (A)–(F) are radiographs of the lateral view of prepared hemi-mandibles taken from pet rabbits at different stages of ADD. The radiographs were taken at the same exposure.
(A) A hemi-mandible and cheek teeth of a pet rabbit unaffected by dental disease (Grade 1, see Box 5.1). The rabbit was kept outside under free-range conditions. The lamina dura and calcified structures of the teeth can be seen.
(B) A hemi-mandible of an adult pet rabbit in the initial stages of ADD. There is loss of supporting alveolar bone, especially at the apex of the tooth. The lamina dura is not visible at the base of the alveolus that has extended into the cortical bone. The structure and shape of the teeth has changed. The enamel cannot be seen on the teeth as clearly as in (A).
(C) A hemi-mandible with the teeth in situ of an adult pet rabbit. The rabbit showed signs of early dental disease (Grade 2, see Box 5.1). Hard swellings could be palpated along the ventral border of the mandible. Radiographically, the enamel on the edge of the teeth and in the central enamel fold is indistinct. The lamina dura is only evident in a few places. The shape of the tooth roots has altered although the occlusal surfaces are still in alignment. There is widening of the interdental space. There are radiolucent areas of bone, particularly at the apex of the second molar (cheek tooth 4).
(D) A hemi-mandible with the teeth in situ of an adult rabbit with acquired malocclusions (Grade 3, see Box 5.1). The enamel fold is not evident in most of the teeth. Pulp cavities cannot be seen. The teeth have moved and rotated within the sockets. Elongated roots have penetrated the cortical bone. There are areas of radiolucent bone.
(E) The hemi-mandible of a rabbit with advanced dental disease (Grade 5, see Box 5.1). The crowns have broken off and the roots are almost completely resorbed. This hemi-mandible was from an aged rabbit that had spent its life confined to a hutch. It had been eating until shortly before death and was not thin.
(F) The hemi-mandible of an adult rabbit that developed an abscess on the cheek (Grade 5, see Box 5.1). The elongated molar crown had penetrated the buccal mucosa. The crowns of most of the cheek teeth have broken off and the roots have resorbed. There are radiolucent areas of osteomyelitis. The shape of the lower incisor appears relatively normal.
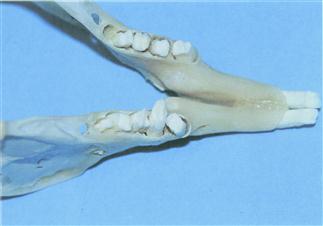
Figure 5.16 Mandible from a rabbit showing early signs of dental disease.
This mandible is from a 2-year-old castrated dwarf lop house rabbit that died due to intestinal obstruction caused by a felt of ingested hair. Although swellings could be palpated along the ventral border of the mandible, no other signs of dental disease were evident. The crowns were not long. The structure, shape and position of the teeth have changed so they are no longer in alignment. Loss of alveolar bone has resulted in wide periodontal spaces. The second right premolar is starting to tip towards the tongue.
In domestic animals metabolic bone disease is usually nutritional. The term ‘nutritional secondary hyperparathyroidism’ is often used. Nutritional secondary hyperparathyroidism can be caused by insufficient dietary calcium or a relative excess of phosphate. Vitamin D plays an integral part because of its effects on intestinal absorption, renal excretion and the mobilization of calcium to and from bone. There is a complex inter-relationship between calcium, phosphorus, vitamin D, parathyroid hormone (PTH) and calcitonin that maintains calcium homeostasis. PTH is released from the parathyroid glands, in response to a fall in blood calcium concentrations. PTH stimulates bone resorption and conversion of 25-hydroxycholecalciferol (25-OH-D) to dihydroxycholecalciferol (1,25(OH)2D) in the kidney. A rise in PTH concentrations indicates the presence of metabolic bone disease. Radiography is an important part of diagnosis of metabolic bone disease. The radiographic signs of nutritional secondary hyperparathyroidism in any species have been described and include resorption of the cortex of the tooth socket (lamina dura), osteoporosis of all bones, especially the calvarium and mandible, folding fractures of the long bones, compression fractures of the vertebrae and abnormalities of the pelvis (Morgan, 1972). Many of the radiographic changes that take place in rabbits with ADD are typical of metabolic bone disease. Resorption of the lamina dura is seen, especially on post-mortem radiographs of hemi-mandibles (see Figure 5.15) and osteoporosis of the mandible and calvarium are characteristic findings in prepared skulls (see Figure 5.11).
Rabbits have an unusual calcium metabolism (see Section 1.3.12). They absorb dietary calcium efficiently from the gut and excrete large quantities in the urine. However, despite their efficient absorption, rabbits do have a minimum requirement for calcium (Chapin and Smith, 1967) and the diet of pet rabbits can be calcium deficient. Mixed rations permit selection of calcium-deficient items such as maize and peas and rejection of the vitamin and mineral supplement. The supplement is usually incorporated into pellets that are not as palatable to rabbits as the other ingredients (see Figure 1.8). Analysis of the selected diet has shown that it can be severely deficient in calcium (see Table 1.3) (Harcourt-Brown, 1996). Selective feeding has been reported as a cause of metabolic bone disease in other species. For example, selection of cereals and bread from a mixed diet including pellets resulted in fractured vertebrae in the necks of emus and rheas (Wolf et al., 1996). Dietary calcium deficiency has been shown to cause poor mineralization of the skeleton in rabbits. Laboratory rabbits are used as models of human osteoporosis. Interestingly, osteoporosis in laboratory rabbits is induced by neutering females and administering steroids. Whether dental disease is found in these rabbits is not reported; however, jaw osteoporosis has been demonstrated. Reduced bone density can be induced by feeding a calcium-deficient diet (Gilsanz et al., 1991). In a study by Wu et al. (1990), mature rabbits suffered a 20% vertebral bone loss after only 14 weeks on a calcium-deficient diet.
In addition to its inter-relationship with PTH and blood calcium levels, vitamin D also has a direct effect on the structure of teeth and in modelling and remodelling of bone. Fracture healing in laboratory rabbits is improved with vitamin D supplementation (Omeroglu et al., 1997) and vitamin D influences the organization and mineralization of rabbit cartilage in vitro (Plachot et al., 1982). The direct effect of vitamin D on bone and tooth structure could also be a factor in the development of dental disease in rabbits. In humans, vitamin D-resistant rickets is associated with defective calcification of the teeth and periapical infections (Archard, 1971; Goodman et al., 1998).
Radiographs of rabbit mandibles taken during a study of ‘calcification processes’ by Mellanby and Killick (1926) showed demineralization of the bone and root elongation in rabbits fed on a diet that would induce rickets. The changes showed a great similarity to the changes seen in the mandibles of present-day pet rabbits suffering from ADD.
In rabbits, although vitamin D does not appear to be required for intestinal absorption of calcium if there is sufficient quantity in the diet, vitamin D is required to increase intestinal absorption if dietary calcium concentrations are restricted (Brommage et al., 1988). Vitamin D will increase intestinal absorption of calcium in rabbits (Tvedegaard, 1987). Rabbits kept indoors or housed in hutches and carports away from sunlight cannot synthesize endogenous vitamin D. Supplemented vitamin D is not ingested by rabbits that leave the pellet portion of mixed rations uneaten. Blood vitamin D assays have demonstrated undetectable 1,25(OH)2D3 concentrations in pet rabbits, especially in samples taken during the spring (Fairham and Harcourt-Brown, 1999).
Hay that has been artificially dried without exposure to sunlight may not contain much vitamin D. Calcium can also be leached out of poor-quality hay. Calcium levels in hay can be as low as 0.25%, which is below the 0.44% required for bone calcification in rabbits (Chapin and Smith, 1967; McDonald et al., 1996).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


