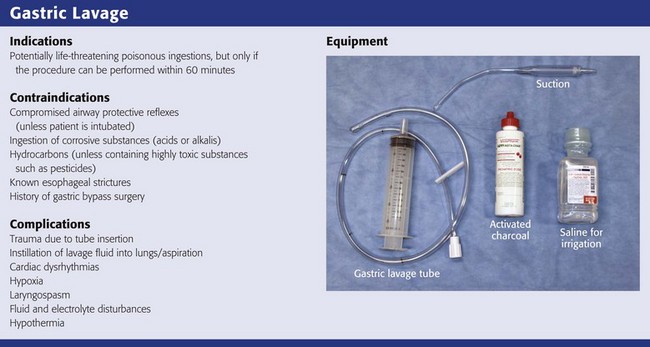
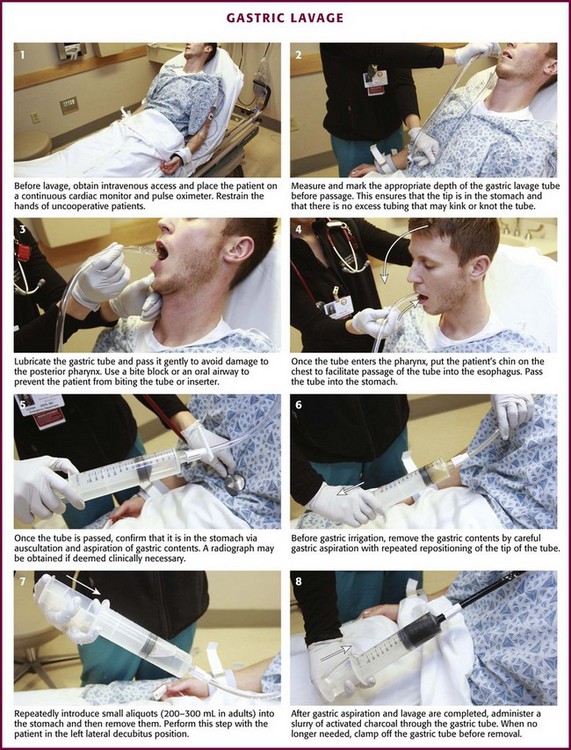
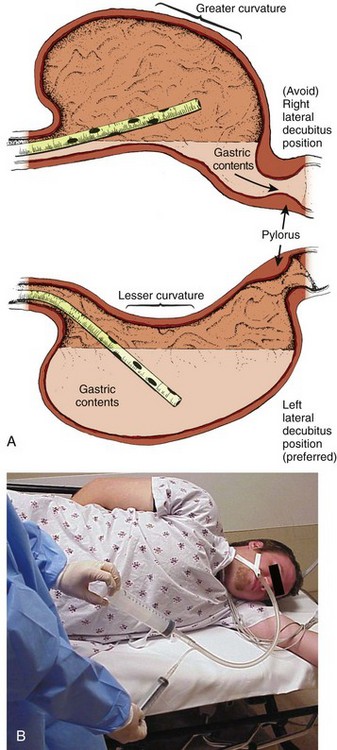
Chapter 42 In 2009, the National Poison Data System of the American Association of Poison Control Centers reported 2,479,355 human toxic exposures and 1452 resultant fatalities.1 Of these total exposures, 24.1% were managed in a health care facility. Massive exposure to some toxic agents (e.g., calcium channel blockers, tricyclic and other antidepressants, antipsychotics, β-blockers, colchicine, chloroquine, cyanide, Amanita phalloides mushrooms, paraquat) will probably result in severe morbidity or fatality regardless of even the most sophisticated and timely medical interventions. With general supportive care and the use of a few specific antidotes, however, the mortality rate in unselected overdose patients is less than 1% if the patient arrives at the hospital in time for the clinician to intervene. Key management of poisoned patients seen in health care facilities initially focuses on confirming the diagnosis of possible exposure to a toxin, providing standard cardiovascular and respiratory supportive care, and using a small cadre of specific antidotes. In rare and yet undefined selected instances, prevention of further absorption of toxin by various decontamination procedures may theoretically ameliorate the morbidity or reduce mortality. Although a better final outcome after gastric decontamination may seem intuitively reasonable, there is no definitive evidence from prospective clinical trials that the use of various decontamination techniques positively alters the morbidity or mortality of a poisoned patient.2 Before the availability of objective or experimental evidence addressing gastric-emptying procedures, most clinicians instituted previously performed unproven decontamination procedures in the emergency department (ED) as a reflex response for the majority of patients suspected of drug overdose, often without much forethought and certainly without confirming data. Mounting evidence relegates any benefit from any form of gastric decontamination to selected cases and specific individual scenarios. In fact, because of the lack of demonstrable benefit and the mounting evidence for potential harm, syrup of ipecac, once a mainstay in the management of poisonings, is no longer recommended; the American Heart Association and American Red Cross International Consensus on First Aid Science state that syrup of ipecac should not be used in first aid treatment of acute poisonings,3 and parents have been instructed by the American Academy of Pediatrics to remove it from the home.4 Parents and health care providers appear to be heeding this recommendation since less than 0.01% of poisoned patients received ipecac in 2009.1 The use of gastric lavage in poisoned patients has decreased significantly since the 1990s, and in the United States it is currently reported to be used in less than 1% of overdose case.1,5,6 Numerous animal and human volunteer studies have been conducted to examine the effectiveness of gastric lavage in removing toxins from the stomach, especially with respect to other gastrointestinal decontamination methods.7–18 The reported efficacy of gastric lavage in removing markers from the stomach varies significantly in these studies. The difference in these study results is due in part to the variability of the methods used (different fluid-instilled markers, animal models, positioning, amount of lavage, and lavage tube size) and the time that elapsed from instillation of the marker in the stomach until gastric lavage was performed. Even within individual studies, the range of effectiveness of gastric lavage in removing the marker varied considerably. For example, Tandberg and coworkers performed gastric lavage 10 minutes after ingestion of the marker and reported that its effectiveness in removing the marker varied from 18.9% to 67.7%.13 Many of these studies do not replicate the typical clinical scenario encountered in emergency medicine.2,19 The efficiency of gastric lavage in removing a marker significantly decreases with increasing time after ingestion. This is due to the fact that as time increases after ingestion, there is more time for the marker to be absorbed and pass out of the stomach. For example, Shrestha and colleagues reported that more than 70% of the marker used in their study passed out of the stomach by 60 minutes.20 It is rare that gastric lavage can be performed within the first hour after toxic ingestion. Not only does it take time for these patients to arrive at the ED, but it also takes time for evaluation, stabilization, and performance of gastric lavage. Watson and associates reported that the mean time required by experienced emergency medicine nurses to perform lavage was 1.3 hours.16 Gastric lavage may also propel the marker from the stomach into the small intestine, thereby decreasing the effectiveness of removing the toxin from the stomach and actually enhancing the rate of absorption.21 Three major studies have examined whether gastric lavage positively influences the outcome of poisoned patients.22–24 In a study performed by Kulig and coworkers, there was no difference in outcome in patients who received gastric lavage followed by charcoal versus charcoal alone when these interventions were performed more than 1 hour after ingestion.22 In patients who were treated within 1 hour of ingestion, gastric lavage followed by charcoal provided a small but statistically significant advantage over activated charcoal alone. Merigian and colleagues demonstrated that in symptomatic patients, the rate of intensive care admission and need for intubation was significantly higher in patients who received gastric lavage followed by charcoal than in those who received charcoal alone.23 This increased admission and intubation rate was directly attributed to the aspiration of gastric contents as a result of gastric lavage. Pond and associates replicated the Kulig and colleagues’ study.24 They found no difference in outcome between patients who received gastric lavage followed by charcoal and those receiving charcoal alone, regardless of the time of performance of gastric lavage. They concluded that “gastric emptying procedures can be omitted from the treatment regimen for adults after acute overdose, including those who present within 1 hour of overdose and those that manifest severe toxicity.” Based on the literature available, gastric lavage should not be routinely used in the management of poisoned patients (Fig. 42-1).25 There is no universally accepted standard of care that can be applied to the use of gastric lavage in unselected poisoned patients in the ED. Under certain circumstances, however, there may be a theoretical benefit from gastric emptying, and the local poison center should be contacted to assist in making a decision whether gastric lavage may be of benefit. Whether specific subsets of overdose patients may benefit from gastric lavage has not been clearly defined. Though generally safe, gastric lavage is not an innocuous procedure. Performance of gastric lavage is contraindicated in any person who demonstrates compromised airway protective reflexes, unless that person is intubated.26 Many clinicians opt for lavage in a seriously ill patient who is intubated because airway protection is already accomplished. Tracheal intubation, however, does not ensure a totally protected airway. Paralyzing plus intubating a patient merely to initiate gastric lavage is generally eschewed. Gastric lavage is contraindicated in persons who have ingested corrosive substances (acids or alkalis) or hydrocarbons (unless they contain highly toxic substances such as pesticides) or have known esophageal strictures or a history of gastric bypass surgery.27 Caution should be exercised in performing gastric lavage in combative patients and in those who have medical conditions such as bleeding diatheses that could be compromised by performing this procedure. If the decision is made to perform gastric lavage, careful attention to the details of the procedure results in increased safety for the patient and more effective removal of the ingested poison. Before lavage, the patient should have intravenous access secured and continuous cardiac monitoring and pulse oximetry initiated (Fig. 42-2, step 1). A large, rigid suction catheter should be available immediately. The position of the patient during gastric lavage is important. Place all patients in the left lateral decubitus, Trendelenburg position (≈20-degree tilt on the table) (Fig. 42-3). This position diminishes the passage of gastric contents into the duodenum during lavage and decreases the risk for pulmonary aspiration of gastric contents should vomiting or retching occur. Restrain the hands of an uncooperative patient to prevent removal of the gastric or endotracheal tube. Intubated patients on a ventilator may be lavaged in the supine position because of logistic reasons (Fig. 42-4). Under no circumstances should a nonintubated patient undergo lavage in the restrained supine position. Such positioning invites aspiration and diminishes the patient’s natural protective maneuvers, such as coughing and sitting up. Figure 42-3 A, Effect of patient positioning on lavage. B, The left lateral decubitus position is preferred. Most clinicians prefer the oral route for gastric lavage, but in selected circumstances a standard large-bore nasogastric (NG) tube (Salem sump pump) may be used. Large-diameter gastric hoses with extra holes cut near the tip have traditionally been recommended for gastric lavage. There are no convincing data on humans to refute or support this recommendation, and one study of a small number of dogs failed to show any difference in efficacy with lavage through a 32-Fr tube versus a 16-Fr lavage tube.28 It is generally held that large-diameter NG or orogastric tubes (>1 cm) are more likely to retrieve particulate matter successfully, but the tube size is such that whole pills are unlikely to pass (Fig. 42-5). Smaller, more flexible tubes may kink and are significantly more difficult to pass. An NG tube may be passed through the mouth or nose, but orogastric hoses should not be passed through the nose. Because most pills disintegrate in the stomach in a few minutes, significant amounts of particulate matter may be retrieved with a large-bore NG tube such as an 18-Fr Salem sump tube. NG tubes are considerably easier to pass and less traumatic for the patient. NG tubes are preferred for liquid ingestions and in children (Fig. 42-6). In most cases, a 36- to 40-Fr or a 30-English gauge tube (external diameter, 12 to 13.3 mm) should be used in adults and a 24- to 28-Fr gauge (diameter, 7.8 to 9.3 mm) tube in children.25 Before passage, estimate the length of tube required to enter the stomach by approximating the distance from the corner of the mouth to the midepigastrium. Premeasurement avoids the curling and kinking of excess hose in the stomach (see Fig. 42-2, step 2). Passage of an excessive length of hose may cause gastric distention, bruising, and perforation, whereas passage of an insufficient length of hose may result in lavage of the esophagus and increased risk for emesis and aspiration. Commercial lavage systems are available and often use either a gravity fill-and-empty system with a Y connector or a closed irrigation syringe system. Alternatively, an irrigation syringe can be used for intermittent input and withdrawal of lavage fluid. Lubricate the gastric tube and pass it gently to avoid damage to the posterior pharynx (see Fig. 42-2, step 3). Use of a bite block or an oral airway may prevent the patient from chewing on the orogastric tube and biting the fingers of the inserter. If the patient is obtunded or paralyzed, extend the jaw to facilitate passage. Never use force to pass the tube. Once the pharynx has been entered, put the patient’s chin on the chest to facilitate passage of the tube into the esophagus (see Fig. 42-2, step 4). Cough, stridor, or cyanosis indicates that the tube has entered the trachea; withdraw the tube immediately and reattempt passage. Once the tube is passed, confirm that it is in the stomach. Intragastric placement is usually evident on clinical grounds by the spontaneous egress of gastric contents but may be confirmed by auscultation of the stomach during injection of air with a 50-mL syringe followed by successful aspiration of gastric contents (see Fig. 42-2, step 5). In an intubated or obtunded patient or a young child, confirm tube position radiographically before lavaging, although this is not routinely performed (Fig. 42-7A). A misplaced tube may irrigate the esophagus with a tube that has doubled back on itself during passage (see Fig. 42-7B). The most serious complication, other than esophageal perforation, is inadvertent passage of the tube into the lungs. Tracheal passage of a lavage tube should be readily obvious in an awake patient before lavage, and obtunded patients are intubated, thereby obviating this problem. If an awake patient begins to vomit during lavage, immediately remove the tube to allow the patient to protect the airway. Before gastric irrigation, remove the gastric contents by careful gastric aspiration with repeated repositioning of the tip of the tube (see Fig. 42-2, step 6). With the Y-connector closed system, perform lavage by clamping the drainage arm of the Y adapter and infusing aliquots of fluid into the stomach from a reservoir. Clamp the reservoir arm of the Y, and then open the drainage arm to permit drainage of the stomach contents by gravity. Repeat this procedure. Some resistance is produced by the Y connector and tubing. Apply suction intermittently to the drainage tubing to enhance emptying of the stomach. Lavage can be performed adequately with tap water in adults. Because electrolyte disturbances have occurred in children who underwent lavage with tap water, prewarmed (45°C) normal saline is generally recommended for children.29–31 Warmed lavage fluid increases the solubility of most substances, delays gastric emptying, and should theoretically increase the effectiveness of the procedure.32,33 Repeatedly introduce small aliquots of lavage solution (200 to 300 mL in adults and 10 mL/kg body weight in children up to a maximum of 300 mL) into the stomach and then remove them (see Fig. 42-2, step 7). Larger amounts of fluid create the potential for an increased risk of washing the gastric contents into the duodenum or lungs. Much smaller amounts are not clinically practical because of the dead space in the tubing (≈50 mL in a 36-Fr hose) and the increased time required. The amount of fluid that is returned should approximate the amount introduced. Manual agitation of the patient’s stomach by gently “kneading” it with a hand placed on the abdomen may increase recovery.32 Continue lavage until the fluid becomes clear. After gastric aspiration and lavage have been completed, administer a slurry of activated charcoal through the gastric tube (see Fig. 42-2, step 8). When no longer needed, clamp off the gastric tube during removal to avoid “dribbling” fluid into the airway. With the increasing use of repetitive doses of activated charcoal, an NG tube may be left in place, or passed, after the lavage procedure is completed. A patient who remains obtunded may receive additional doses via a standard NG tube. Because the large gastric tube is irritating and may predispose the patient to gagging, drooling, or aspiration, it should be removed. Alert patients should take subsequent doses of charcoal orally as necessary. A correctly performed procedure in the appropriate environment is generally safe, but numerous complications have been associated with gastric lavage.26 The complications can be divided into those caused by mechanical trauma and those resulting from the lavage fluid. Depending on the route selected for insertion of the tube, damage to the nasal mucosa, turbinates, pharynx, esophagus, and stomach has been reported.34–37 After insertion of the tube, it is imperative to confirm correct placement. Scalzo and associates found radiographically that 7 of 14 children had improper tube placement (too high or too low) despite positive gastric auscultation in all cases, although the clinical effects of such misplacement was not evaluated. Radiographic confirmation of tube placement should be considered in young children and intubated patients (see Fig. 42-7).38 Instillation of lavage fluid and charcoal into the lungs through tubes inadvertently misplaced within the airways has been reported.39 During lavage, changes in cardiorespiratory function have been noted. Thompson and coworkers reported that during lavage 36% of patients had atrial or ventricular ectopy, 4.8% had transient ST elevation, and 29% had a fall in oxygen tension to 60 mm Hg or lower.40 Laryngospasm may also occur during gastric lavage.25 The lavage fluid itself is a potential source of complications. The large amount of fluid administered during lavage has been reported to cause fluid and electrolyte disturbances. These disturbances have been seen with the use of both hypertonic and hypotonic lavage fluid in the pediatric population.29–31 Hypothermia is a possible complication if the lavage fluid is not prewarmed. Pulmonary aspiration of gastric contents or lavage fluid is the primary potential risk during gastric lavage, especially in patients with compromised airway protective reflexes.41–43 Merigian and colleagues reported a 10% incidence of aspiration pneumonia in patients who underwent gastric lavage.23 This risk is reduced by using small aliquots of lavage fluid, adequately positioning the patient, and intubating patients with compromised airway protective reflexes. If the lavage tube cannot be removed easily, do not force it. Kinking or knotting of the tube can occur, but occasionally a tube may become stuck because of lower esophageal spasm. If fluoroscopy or radiography demonstrates no deformation of the lavage tube, 1 to 2 mg of intravenous glucagon can be infused in an attempt to relieve lower esophageal spasm.44 Surgical removal may be necessary if the gastric tube is deformed by kinking or knotting. Activated charcoal is a carbon product that is subjected to heat and oxidized to increase its surface area (Fig. 42-8). It has the capacity to adsorb substances onto the porous surface of the charcoal. The use of activated charcoal for poisoning has been recognized for almost 2 centuries. To demonstrate charcoal’s effectiveness, in 1930 French pharmacist Touery ingested several times the lethal dose of strychnine mixed with 15 g of activated charcoal. He performed this act in front of a class of colleagues and exhibited no ill effects. Activated charcoal acts both by adsorbing a wide range of toxins present in the gastrointestinal tract and by enhancing elimination of toxin if systemic absorption has already occurred. It enhances elimination by creating a concentration gradient between the contents of the bowel and the circulation, but it also has the potential of interrupting enterohepatic circulation if the particular toxin is secreted in bile and enters the gastrointestinal tract before reabsorption.45 Oral activated charcoal is given as a single dose or in multiple doses. The adsorptive capacity of charcoal depends on the inherent properties of the toxin and the local milieu, such as pH. Adsorption begins within minutes of contact with a toxin but may not reach equilibrium for 20 to 30 minutes. Desorption of toxins from charcoal occurs over time, although this has little clinical significance for most patients and can be overcome by administering additional charcoal. For years, administration of a single oral dose of activated charcoal for essentially all overdoses has been routine. However, with the emergence of new guidelines in overdose management,6 use of charcoal has declined to less than 5% of all potential toxin exposures (Fig. 42-9).1 Clearly, charcoal binds many toxins in the gut, thereby decreasing some systemic absorption. Despite a lack of scientific data demonstrating a decrease in morbidity and mortality and firm evidence to support its widespread use, charcoal is a reasonable intervention for most poisoned patients encountered in the ED if it can be administered easily and safely. The exact indications are not established, and no universally accepted standard of care has been promulgated.46 A single dose of activated charcoal is indicated if the clinician estimates that a clinically significant fraction of the ingested substance remains in the gastrointestinal tract, the toxin is adsorbed by charcoal, further absorption may result in clinical deterioration, and it can be administered safely. This will usually be a clinical decision because adequate historical data may often be lacking. It may also be administered by multiple dosing if the clinician anticipates that the charcoal will result in increased clearance of an already absorbed drug. It is most effective within the first 60 minutes after an oral overdose and decreases in effectiveness over time. Charcoal is generally considered to provide superior gut decontamination over gastric lavage. There is no definitive evidence, however, that administration of activated charcoal improves outcome. Administration of charcoal is contraindicated in any person who demonstrates compromised airway protective reflexes unless already intubated.46 It is absolutely contraindicated in persons who have ingested corrosive substances (acids or alkalis). Not only does charcoal provide no benefit in a corrosive ingestion, but its administration could also precipitate vomiting, obscure endoscopic visualization, and lead to complications if a perforation developed and charcoal entered the mediastinum, peritoneum, or pleural space. Charcoal should be avoided with ingestion of a pure aliphatic petroleum distillate. Hydrocarbons are not well adsorbed by activated charcoal, and its administration could lead to further risk for aspiration. Many hydrocarbons are potential systemic toxins (e.g., carbon tetrachloride and benzene) or are mixed with other potentially significant toxins such as pesticides. In these cases data are lacking, but charcoal administration can be considered. Caution should be exercised in using charcoal in patients with medical conditions that could be further compromised by charcoal ingestion, such as gastrointestinal perforation or bleeding. Charcoal is not indicated for isolated ingestion of ethanol or metals (e.g., iron, lithium) because these substances are not adsorbed. If the airway is not secure, charcoal should be given with caution to minimally symptomatic patients who have ingested a toxin that may suddenly induce seizures. Because it is often impossible to determine the exact nature of an ingestion, a liberal use policy is advocated for potentially mixed overdoses. Charcoal administration by paramedics and other emergency response personnel should be performed with caution.47 There is insufficient evidence to recommend for or against administration of activated charcoal in the prehospital setting.3 The same indications and contraindications apply as for patients who are in the hospital. The motion of the ambulance during transport may make the patient more prone to emesis. Either spilling of charcoal or vomiting of charcoal may result in significant contamination of the transport vehicle and subsequently place that vehicle out of commission until it can be cleaned.
Decontamination of the Poisoned Patient
Gastric Decontamination
Background
Indications
Contraindications
Equipment and Preparation
Technique
Complications
Activated Charcoal
Indications
Contraindications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Decontamination of the Poisoned Patient