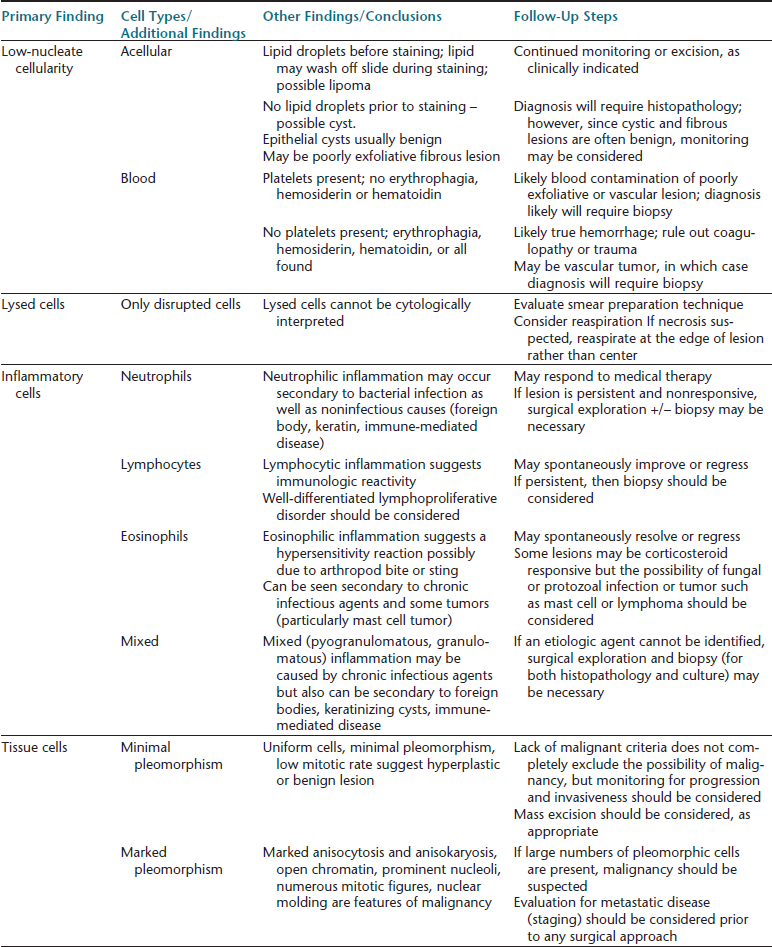
Chapter 5
Cutaneous and Subcutaneous Lesions
Cutaneous and subcutaneous lesions are commonly sampled sites for cytologic evaluation. Several reasons for this are possible, but perhaps some of the primary reasons are that these lesions are easily seen by the owners of the animals and thus cause concern and their sites are easily sampled by veterinarians. For the most part, sampling from skin lesions does not require imaging aid, nor does it necessarily require sedation or anesthesia. Just as important, many lesions are highly exfoliative, and skin or subcutaneous cytologic samples tend to have a high diagnostic yield. In one study comparing cytologic and histopathologic test results of cutaneous and subcutaneous lesions in 243 specimens, diagnosis was in agreement in 90.9% of the cases.1
Despite the potential usefulness of cytologic sampling, it is important to recognize that dermatologic disease encompasses a broad range of inflammatory, noninflammatory, and neoplastic lesions. Not all of these lesions exfoliate well, and many of the diagnoses are dependent on tissue distribution of cells, not just cytomorphologic appearance. Cytology interpretation is only based on cells, organisms, and background material, but since these have no architectural arrangement, as can be evaluated in biopsy samples, the results often are less specific compared with histologic interpretation (Figure 5-1). Finally, all cytology samples have some degree of artifact such as smudging of round cells that makes them appear spindloid, thick background material resulting in cells shrinking and appearing more round, or artifactual aggregates of cells resembling cohesive clusters; this artifact may make it difficult to be certain about cell types in some cases.
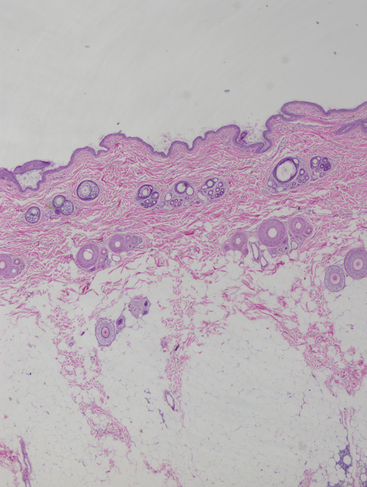
Figure 5-1 Biopsy sample from normal skin.
Skin is divided into three general areas: epidermis, dermis, and hypodermis. Adnexal structures found in skin include sebaceous glands, sweat glands, and hair follicles. The dermis contains the adnexal structures as well as smooth muscle, blood vessels, lymphatic vessels, collagen, and elastic fibers. This histologic section shows adnexal structures in cross-section rather than longitudinally, which is related to tissue processing. The hypodermis (or subcutis) contains adipose tissue and collagen bundles. Histologic diagnoses incorporate specifically what areas of the skin are being affected by inflammatory, degenerative, or neoplastic processes. This is not possible with cytologic methods. (H&E, 2× objective.)
For these reasons, histopathology is often necessary for definitive diagnosis of dermatologic diseases. When taking cytologic samples from skin or subcutaneous lesions, it is useful to explain to the owner that the sampling process, in the best case scenario, will yield a clear-cut diagnosis, failing which, it would be a preliminary step to aid in determining what needs to be done next (Table 5-1).
Collection Techniques
Multiple methods are used for collecting samples for cytologic evaluation. These include needle aspiration, skin swabs, scrapings, and impression smears. Each method has advantages and disadvantages. For mass lesions, the most useful technique is needle aspiration as this allows sampling from a variety of areas in the lesion, including cells found in deeper tissues that are not accessible by other cytologic methods. Many lesions in skin and subcutaneous tissues are highly exfoliative when aspirated, and often a good cell yield is available for interpretation. Fine-needle sampling may be done by aspiration or nonaspiration techniques, depending on the expected degree of exfoliation and the likelihood of blood contamination. If a syringe is used to create negative pressure while the needle is embedded in the lesion of interest, then care must be taken to make sure the pressure is released prior to removing the needle from the mass, as otherwise, the cells may easily be lost into the syringe, or surrounding normal fatty tissue may contaminate the sample. Material collected from solid masses should be carefully expelled onto a slide. A second slide or cover slip should be used to gently spread the material on the first slide into a thin layer. The sample should be allowed to air-dry before being stained with a Romanowsky-type stain. If the lesion is fluid filled, preparation of both direct and sediment smears of the fluid may be useful, but typically, a complete fluid analysis (i.e., protein concentration, cell count, differential in addition to cytology) is not necessary for evaluation, and cytology alone generally will indicate whether an inflammatory, noninflammatory, or neoplastic process is present. If a solid mass is associated with a fluid-filled lesion, then the solid areas as well as the fluid areas of the mass should be sampled.
General Cytologic Evaluation
The approach to cytologic evaluation of skin and subcutaneous lesions is similar to other tissues. Multiple slides should be prepared for review by using standard smear preparation techniques. For the most part, cytology smears need only be stained with a Romanowsky-type stain (Wright, Wright-Giemsa, and quick stains such as Diff-Quik). In some instances, additional stains may be desirable, particularly if it is thought that rare or difficult-to-see microorganisms may be present, in which case, other stains such as acid-fast stains for Mycobacterium or Nocardia and Gomori methenamine silver (GMS) or periodic-acid-Schiff (PAS) stains for fungal organisms may help find these organisms. For this reason, it may be useful to leave some slides unstained in case additional staining is needed. It is possible, however, to destain slides, then restain them with a variety of different special stains and achieve adequate staining quality for interpretation.2
Slides should first be reviewed at low power (4 to 10× objective) to assess the adequacy of the smear. Degree of cellularity, adequate separation of cells, thickness of smear, and good staining quality are some of the factors that should be evaluated. The entire smear area should be reviewed on low power to review for the presence of any large structures such as infrequent cell clusters or large fungal organisms (e.g., Coccidioides spherules) that could be missed with a higher-power lens. The low-power scan should also help identify areas where the cytologist would want to go to a higher-power review. Most cytologic abnormalities may be seen with a good 50× oil immersion objective lens. This lens is preferable to a 40× high-dry lens, although this latter lens is an adequate alternative. The 100× oil lens is needed only to confirm small structures such as bacteria, intercellular granules or inclusions, and small fungal spores or protozoa.
Once the adequacy of the smear has been assessed, a determination may then be made about the pathologic process. On initial review, a definitive diagnosis may be made based purely on the cells, any organisms present, or both. Many times, however, a clear-cut definitive answer is not based on cytology findings alone. In these cases, a systematic approach should be taken to narrow down differentials and, just as importantly, make a decision about what the next diagnostic or therapeutic step needs to be (see Table 5-1). A consistent approach includes a thorough review of all areas of the sample and then systematically classifying the type of pathologic process and likely etiologies. In general, if a mass lesion is aspirated and only tissue cells are collected, then the lesion is caused by either hyperplasia or neoplasia. If only inflammatory cells are present, then the underlying cause may be infectious or noninfectious, keeping in mind that some tumors may elicit an inflammatory response. Mixtures of inflammatory and tissue cells may be difficult to interpret for this reason, in addition to the fact that inflammation may induce morphologic changes in tissue cells.
Inflammatory Cells
Samples with primarily neutrophils are often referred to as displaying neutrophilic, suppurative, or purulent inflammation (Figure 5-2). The neutrophils should be characterized as “well preserved” or “showing some signs of degeneration.” Degenerative changes in neutrophils include nuclear swelling with paler staining chromatin (karyolysis). Severely degenerate neutrophils may have round nuclei, and the cell type may be virtually unrecognizable. Degenerative changes are not pathognomonic for infection but should raise the clinical suspicion of infection. Neutrophilic inflammation is often caused by bacterial infection but may also be seen with fungal or yeast infection as well as noninfectious problems.
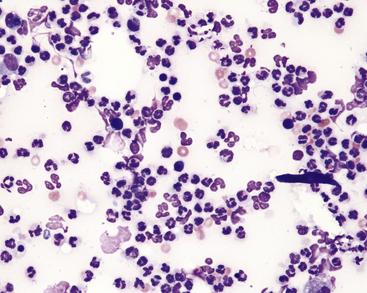
Figure 5-2 Neutrophilic inflammation is characterized by the predominance of neutrophils. (Wright stain.)
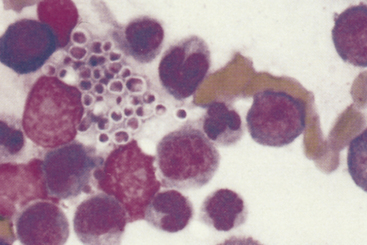
Eosinophilic inflammation (Figure 5-3) in skin has similar considerations as in other tissues. In skin, it is often related to a hypersensitivity response to the bite or sting of an arthropod (e.g., flea, bee, spider, or tick). Other parasites, including mites and nematodes, may also cause eosinophilic inflammation. It is sometimes noted as a component of inflammation secondary to infection, possibly more commonly with fungal infection, and may also be seen as a component of some vaccine reactions. Solid masses or linear plaques with large numbers of eosinophils may be an eosinophilic granuloma, but care must be taken to rule out infectious agents or neoplasia, as an eosinophilic infiltrate may also be seen as a paraneoplastic response to some tumors, most notably mast cell neoplasia as well as others such as lymphoma.
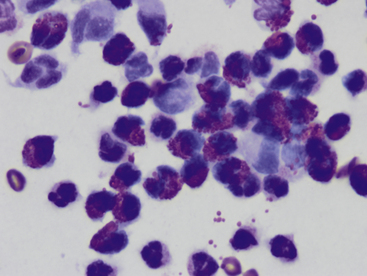
Figure 5-3 Fine-needle aspirate from subcutaneous swelling in a dog.
Mixed inflammatory cells are present, however, the majority are eosinophils. Fewer macrophages and neutrophils are also noted. (Wright-Giemsa, 100× objective.)
Granulomatous inflammation and pyogranulomatous inflammation are terms that should be reserved for histopathologic diagnosis, as these are associated with a specific type of inflammatory cell arrangement in tissues. Nevertheless, these terms are used by some cytologists as well. Others will use chronic inflammation, macrophagic inflammation, or mixed cell inflammation. These terms imply that macrophages are present, with some mixture of lymphocytes and possibly other cells, including sheets of epithelioid macrophages and multinucleate cells (Figure 5-4). This type of inflammation typically is associated with chronic inflammatory disorders, and these often involve chronic infectious agents such as higher bacteria (Actinomyces, Nocardia, Mycobacterium spp.), fungal or protozoal organisms, or noninfectious agents such as foreign bodies. If nothing else, this type of inflammation indicates the lesion may be unresponsive to routine antibiotic therapy alone.
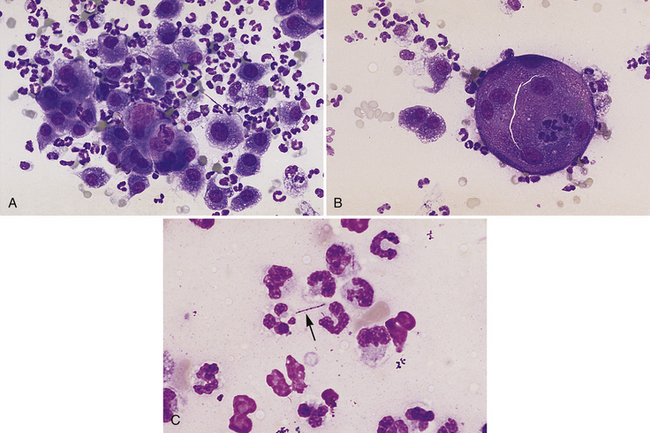
Figure 5-4 Impression smears of a pyogranulomatous nodule in a dog.
A, Pyogranulomatous inflammation includes a mixture of neutrophils and macrophages with the macrophages often noted in sheets. (Diff-Quik, original magnification 160×.) B, Multinucleate inflammatory giant cell with scattered neutrophils and macrophages from the same lesion shown in A. (Diff-Quik, original magnification 132×). C, Long, slender, filamentous bacterial rod (arrow) present among inflammatory cells. (Diff-Quik, original magnification 400×).
Infectious Agents
Bacteria
Superficial or deep pyoderma and bacterial abscesses
Surface or superficial pyoderma presents with skin surface abnormalities, including pustules, papules, crusts, and erythema. Bacteria may be identified with impression smears or scrapings, but these techniques potentially could miss other underlying disease. Deeper pyoderma results from infection extending deeper into follicular tissue, resulting in nodules and abscesses. The most common bacterial pathogen for superficial and deep pyoderma is Staphylococcus intermedius (Figure 5-5, A); however, other secondary invaders may also exist, and in chronic cases, bacterial culture and sensitivity may be advisable.3 Bacterial infection may also occur as a consequence of a bite or other penetrating wound. These often result in a mixed infection with both aerobic and anaerobic organisms (see Figure 5-5, B).
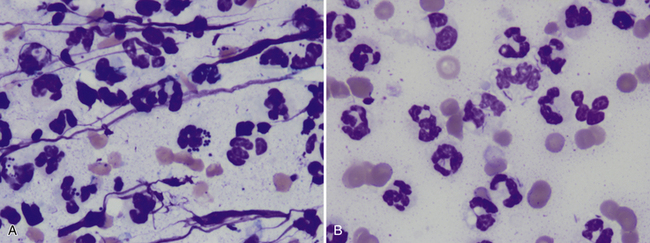
Figure 5-5 Samples from areas with septic inflammation.
A, Most cells are degenerating neutrophils. Some contain phagocytized bacterial cocci. Streaming nuclear material is also present in addition to scattered erythrocytes. (Wright-Giemsa stain, 100× objective.) B, This sample also contains many degenerating neutrophils as well as erythrocytes. Many of the neutrophils contain thin rods in the cytoplasm. Some bacteria appear to be free in the background. The organisms suggest the infection is secondary to a bite wound, foreign body, or other penetrating wound. (Wright-Giemsa, 100× objective.)
Mycobacterium Spp
Mycobacterial infection manifests in several different forms, including slow-growing tuberculous; lepromatous, cutaneous nodular; slow-growing, nontuberculous, cutaneous; and fast-growing, cutaneous and subcutaneous. All of these forms may result in cutaneous lesions. Disseminated infection may also occur particularly with tuberculous disease. Cutaneous lesions include draining nodules, ulceration, and nodules.3 Definitive identification of organisms requires specialized culture for both rapid growers and slow growers. In some cases, polymerase chain reaction (PCR) testing of tissue samples may be helpful.
In cytologic samples, Mycobacterium spp. on rare occasions may be seen as filamentous organisms but are more commonly found as short rods. Mycobacterial organisms do not stain with Romanowsky stains because of their thick lipid walls. They still may be readily identifiable on Romanowsky-stained slides as nonstaining short rods, often noted in macrophages (Figure 5-6). These organisms will stain positive with an acid-fast stain.
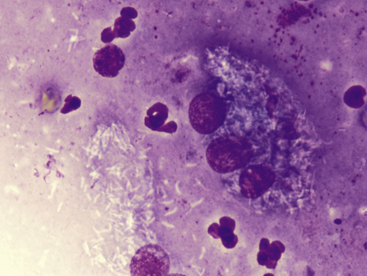
Figure 5-6 Aspirate from a canine leproid granuloma.
Large numbers of nonstaining short bacterial rods (mycobacteria) are seen within a multinucleate cell. Numerous rods are also free in the background, as well as scattered neutrophils and free nuclear material. The nonstaining rods must be distinguished from artifact or other debris and can be confirmed as bacterial organisms with an acid-fast stain (not shown). (Wright-Giemsa, 100× objective.)
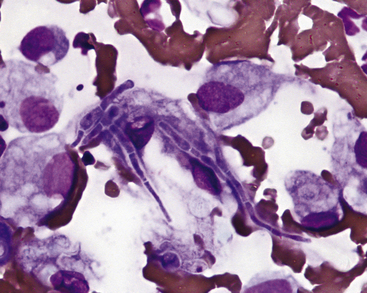
Actinomyces Spp. and Nocardia Spp
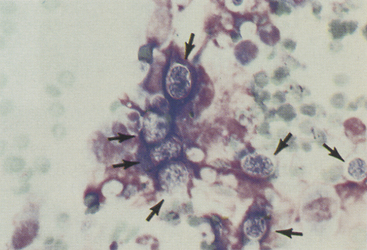
These organisms are often associated with chronic draining or nodular lesions. Infection is typically secondary to a bite wound or penetrating foreign body. These filamentous bacteria are often branching and may be found in thick mats on smear preparations. Actinomyces and Nocardia spp. sometimes stain poorly with Romanowsky stains and may only be noted as a faint outline, often best visualized in the cytoplasm of epithelioid macrophages (Figure 5-7, A). When stained, they are light blue in color with an intermittent pink beading appearance. Nocardia spp. also may be partially acid-fast positive, which may help distinguish them from Actinomyces spp. (see Figure 5-7, B). Culture is required to definitively identify these organisms.
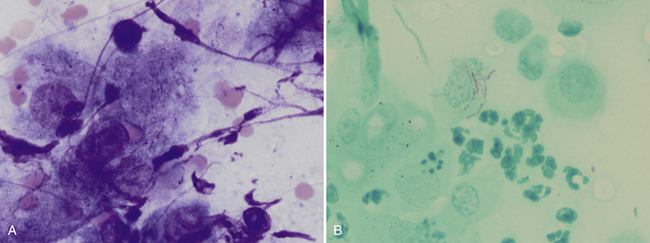
Figure 5-7 Fine-needle aspirate from a draining nodule in a cat.
A, Large epithelioid macrophages or spindle cells mixed with degenerating cells and nuclear debris. Thin, beaded, branching bacterial rods are present and particularly evident in the cytoplasm of the central cell. (Wright-Giemsa, 100× objective.) B, Slide stained with acid-fast stain. Note pink staining thin branching organisms in cell cytoplasm supporting the diagnosis of Nocardia spp. infection. Mycobacterial organisms more typically are short rods but, on rare occasions, may be filamentous, and definitive identification as Nocardia requires culture. Actinomyces spp. may appear morphologically similar as in A but do not stain with acid-fast stain. (100× objective).
Yeast, Fungi, and Algae
Malassezia pachydermatitis
Overgrowth of these organisms typically occurs on the skin surface and usually is not associated with a significant exudative process. These yeast are small (3-8 micrometers [µm] diameter) and typically shaped like a “footprint” (Figure 5-8). They are considered normal flora of skin, so just finding a few does not necessarily indicate abnormality. Greater than two organisms per 100× field has been suggested as indicative of overgrowth.3 It is not unusual to find large numbers of organisms as well as large numbers of anucleate squames but minimal to no evidence for inflammation. Overgrowth of these organisms is usually thought to be secondary to other diseases such as skin allergies, seborrhea, bacterial pyoderma, and skin conformation problems or secondary to antibiotic use.
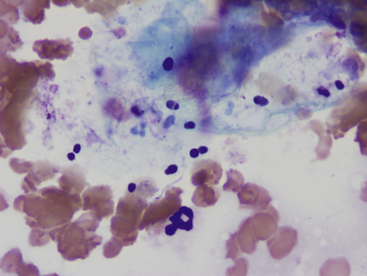
Figure 5-8 Malassezia pachydermatitis from a skin lesion on a dog.
Numerous small oval to “footprint” shaped organisms are noted associated with squamous debris. Large numbers of erythrocytes are also present, as well as a single neutrophil in the lower center region. (Wright-Giemsa, 100× objective.)
Dermatophytes
Dermatophyte infections may manifest in varying ways. In dogs, infection typically manifests as expanding annular areas of alopecia, scales, and crusts. Infection may be localized but may also occasionally be seen with extensive skin involvement. On occasion, infection may result in a solid, raised, nodular lesion, referred to as a kerion. In cats, infection usually causes one or more areas of annular to irregular alopecia with or without scales. Skin impressions or scrapings from the edge of annular lesions typically will demonstrate cellular debris and mixed inflammation, including neutrophils, macrophages, lymphocytes, and eosinophils. Accumulations of hair follicle structures and, particularly around these areas, small fungal spores may be found, sometimes in dense aggregates (Figure 5-9). On rare occasions, septate hyphae may be found. Similar findings may be noted with aspiration of kerion lesions. The causative agents for dermatophytosis are most commonly Microsporum canis, M. gyseum, and Trichophyton mentagrophytes. Fungal culture is required to definitively identify the organism.
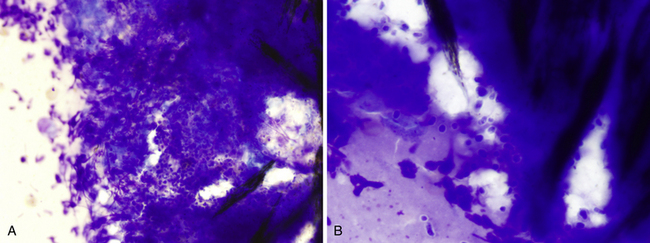
Figure 5-9 Aspirate from a dermatophytic kerion in a dog.
A, Large numbers of small round to slightly oval fungal spores are in the center of the figure. The spores have a small nonstaining capsule and are surrounded by a large amount of nuclear material primarily from degenerating neutrophils. Small fragments of hair are noted at the right. (Wright-Giemsa, 50× objective.) B, Same lesion with 100× objective. Note the mild variation in spore shape, as well as the diffuse stippled internal appearance. (Wright-Giemsa.)
Histoplasma capsulatum
Infection with this organism typically causes systemic disease, and animals may present with a variety of clinical signs, including fever, weight loss, anorexia, coughing, and gastrointestinal signs. Infection may also cause cutaneous lesions that are characterized by papules, nodules, ulcers, and draining tracts. Impression smears from oozing tracts or fine-needle aspirate of nodular lesions reveal mixed inflammation with variable numbers of small (2-4 µm diameter) round yeast, with a thin nonstaining capsule and a basophilic nucleus that often is crescent shaped (Figure 5-10). These organisms may be noted free in the background and may be phagocytized by macrophages as well as neutrophils.
Sporothrix schenckii
Infection with this agent may cause cutaneous, cutaneolymphatic, or disseminated lesions. In dogs, cutaneous lesions often occur on the head or trunk and typically present as nodules. These may ulcerate with purulent discharge and crusts. In cats, draining tracts are typically noted on distal limbs, head, or tail base. Samples from these sites typically reveal neutrophilic to pyogranulomatous inflammation, with variable numbers of yeast organisms noted in the macrophages, in the neutrophils, and in the background. In particular, cats often will have large numbers of organisms in discharge material, and for this reason, cats with this infection need to be handled with caution to prevent zoonotic spread. The yeast organisms are pleomorphic in shape, varying from round to oval to cigar shape, 2 to 10 µm in length, and 1 to 3 µm wide (Figure 5-11). Although similar in size to Histoplasma, Sporothrix may be distinguished by its variety of shapes.
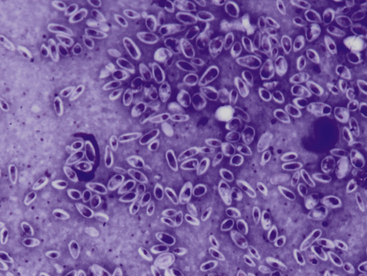
Figure 5-11 Impression smear from a draining lesion in a cat with Sporothrix schenckii infection.
Large numbers of small pleomorphic yeast are present. The yeast vary from round to cigar shaped with a thin, nonstaining capsule. A small lymphocyte is seen to the right. (Wright-Giemsa, 100× objective.)
Blastomyces dermatitidis
Clinical signs associated with blastomycosis include anorexia, weight loss, cough, ocular disease, lameness, and skin lesions. Skin lesions may be found in up to 40% of cases.3 These lesions include firm papules, nodules, plaques, ulcers, draining tracts, and abscesses. Material for cytologic evaluation may be collected from draining tracts or aspirated from nodules or abscesses. These samples reveal neutrophilic to pyogranulomatous inflammation with variable numbers of organisms. These are dimorphic fungus, which are typically found in the yeast phase in infected animals, although on rare occasion may form hyphae in tissues.4 The yeast organisms are variably sized (5-20 µm in diameter), with a thick, blue wall and a thin, nonstaining capsule (Figure 5-12). Occasionally, broad-based budding will be seen, which is useful in distinguishing from Cryptococcus. Blastomyces are larger than Histoplasma and Sporothrix, but typically smaller than Coccidiodes spherules.
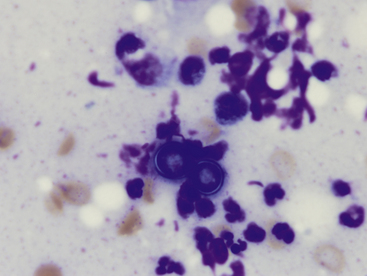
Figure 5-12 Two Blastomyces dermatitidis yeast.
The yeast are adjacent to one another. When budding is present (not shown), it is thick and broad based. The organisms tend to be uniform in size, stain deep stippled blue, and have a thin nonstaining capsule. Scattered degenerating neutrophils, lymphocytes, macrophages, and nuclear debris are also present. (Wright-Giemsa, 100× objective.)
Cryptococcus Spp
Cryptococcosis is most commonly associated with nasal signs (sneezing, snuffling, nasal discharge) in cats and central nervous system or ocular signs in dogs. Both species may also have skin lesions, which, however, are more common in cats. The skin lesions may occur as solitary lesions or as part of disseminated disease. Lesions include papules, nodules, ulcers, abscesses, and draining tracts. Sampling from these lesions often reveals large numbers of organisms. These are yeast organisms that are round in shape (occasionally fusiform) and variably sized (2-20 µm in diameter). The organisms stain pale pink to purple and often have a granular appearance. They typically have a large nonstaining capsule, which may be up to 40 µm in diameter (Figure 5-13). Sometimes, only smaller yeast organisms with thinner capsules are found, and thus, the lack of a thick capsule should not be used to rule out Cryptococcus spp. infection. On occasion, narrow-based budding, which is a useful diagnostic feature, may be seen. Minimal associated inflammation may be present, in which case, it is typically granulomatous. The etiologic agent is typically Cryptococcus neoformans; however, Cryptococcus gattii has emerged as another pathogen occurring in the tropical and subtropical regions of Australia, South America, Southeast Asia, and Africa and also in the North American Pacific Northwest.5 Morphologic features do not distinguish these two species.
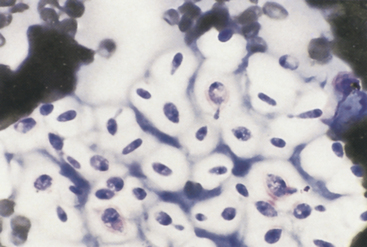
Figure 5-13 Cryptococcus spp. are spherical yeast that frequently have a thick, nonstaining mucoid capsule. Organism size may vary, and the thick capsule is not always present. Narrow-based budding is a characteristic feature. This figure has numerous budding and nonbudding organisms with prominent thick capsules. (Wright stain.)
Coccidioides immitis
Infection with this organism usually causes lung disease but may disseminate systemically and cause skin involvement. Skin lesions may occur as subcutaneous nodules or draining tracts. Cytologic preparations from these lesions typically contain large numbers of inflammatory cells, including neutrophils and macrophages. The organisms are often scarce and not finding an organism does not rule out infection. When present, the organisms are usually found as spherules, which are variable in size but may be large (10-100 µm in diameter) (Figure 5-14). Because of the large size and low numbers of spherules in a sample, these are often easiest to find at low-power (4 to 10×) scans and may be missed if microscopic examination is only done at high power. The spherules have a thick, blue wall with a finely granular internal appearance (endospores). It is often necessary to focus up and down to see the internal detail because of the thickness of the spherules relative to the accompanying cells. On rare occasions, small (2-5 µm) endospores may be seen free in the background or phagocytized by cells. The smaller-sized spherules may be mistaken for Blastomyces, but Coccidiodes spherules do not show budding.
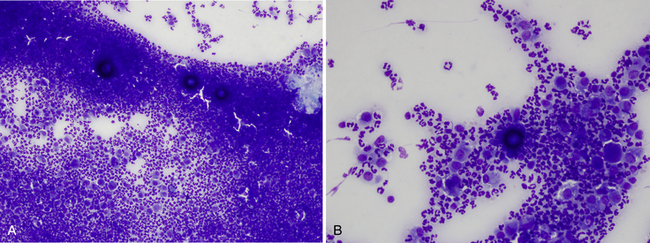
Figure 5-14 Coccidiodes immitis spherules, which are large, spherical structures with a thick wall and stippled blue internal appearance.
A, Low-power magnification from draining cutaneous lesion in a cat. Three large dark-blue spherules are found with marked pyogranulomatous inflammation. The spherules are thick walled and usually keep a three-dimensional shape on cytology smears. Because of this, it is usually not possible to see the spherules sharply in focus in the same plane of focus as the inflammatory cells. Focusing up and down can aid in showing the thick spherule wall as well as the stippled internal appearance. (Wright-Giemsa, 10× objective.) B, Same lesion as A under higher magnification. (Wright-Giemsa, 20× objective.)
Opportunistic Fungi
Many different fungal organisms may infect animals and cause either localized or disseminated skin disease as well as systemic disease. The terminology applied to these infections is complex and beyond the scope of what is possible to diagnose via cytology alone. Fungal skin infections typically present with chronic draining lesions or nodules. Animals with these infections frequently are immunocompromised or have a history of immunosuppressive therapy. Cytologic samples from these lesions will typically reveal pyogranulomatous to granulomatous inflammation. Some cases will also have increased numbers of eosinophils. When organisms are present, usually they occur as hyphal segments (Figure 5-15), although on occasion conidial structures may be found. Categories of opportunistic fungal infection include phaeohyphomycosis (pigmented septate hyphae), hyalohyphomycosis (non-pigmented septate hyphae), mycetoma (localized nodular infections) and zygomycosis (broad, poorly septate hyphae). The structure of the hyphae (septate versus nonseptate; pigmented versus nonpigmented; hyphal width) may be helpful in suggesting a particular species of fungus, but as a rule, fungal culture is necessary to definitively identify the organism.
Pythium insidiosum
Pythium spp. are not true fungi but are water molds that infect dogs more commonly than cats. Infection results in gastrointestinal signs but may also cause cutaneous lesions. Infection usually is thought to occur from standing in or drinking infected water. Cutaneous lesions often involve the extremities and typically are ulcerated nodules with draining tracts. Cytologic samples from these lesions reveal pyogranulomatous inflammation, usually with an eosinophilic component. When organisms are present, they are found as poorly staining, broad, poorly septate hyphae ranging from 2 to 7 µm in diameter. The hyphae do not stain well with Romanowsky stains but are easily visualized with GMS stain (Figure 5-16). A related organism that also may cause skin infections, Lagenidium sp., has hyphae with broader (diameter ranging from 25 to 40 µm) and more irregular or bulbous hyphae than Pythium. Definitive identification of these organisms may require a combination of fungal culture, serology testing, and biopsy with immunohistochemical testing, other molecular diagnostics, or both.3
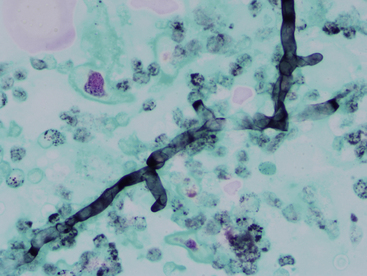
Figure 5-16 Pythium hyphal element.
These organisms have poorly septated, branching hyphae with relatively parallel walls ranging in diameter from 2 to 7 micrometers. The hyphae stain poorly or not at all with Romanowsky stain and are easy to miss with routine cytologic staining. If infection is suspected, then Gomori methenamine silver (GMS) staining should be performed. Periodic acid-Schiff (PAS) does not stain this organism. (GMS, 50× objective.)
Prototheca Spp
Prototheca spp., which are colorless algae that are ubiquitous in the southern regions of the United States, only rarely cause disease. In dogs, infection is often disseminated, but only cutaneous protothecosis has been reported in cats.3 Cutaneous protothecosis is caused by Prototheca wickerhamii in both dogs and cats, whereas disseminated infections in dogs is almost always caused by Prototheca zopfii. Dermatologic lesions include multiple papules and nodules, often over pressure points, or nodules and ulcers involving the mucocutaneous junctions (especially nostrils), scrotum, and footpads. Samples from these lesions reveal pyogranulomatous to granulomatous inflammation, with few to numerous organisms. The organisms are found as spherules or cells (Figure 5-17), which are round to oval and 2 to 30 µm in diameter. The organisms reproduce by endosporulation, and most commonly, two to four (occasionally up to 20) endospores are visible inside the spherule. Most organisms are extracellular, but smaller organisms may be phagocytized by macrophages or neutrophils.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree