Marco Antonio Alvarenga, Frederico Ozanam Papa, Carlos Ramires Neto
Cryopreservation of Stallion Semen
Multiple improvements have been achieved in the past 10 years with regard to utilization and processing of frozen stallion semen. The most remarkable advances include use of new artificial insemination (AI) techniques, such as deep uterine AI, that permit use of a small number of sperm cells; use of alternative cryoprotectors and new commercially available extenders that allow better cell protection during freezing; and use of sperm selection techniques that also increase the quality of frozen semen. With thousands of mares being bred by insemination each year, these improvements will have a positive impact on the use of frozen semen, which affects many breed associations. This chapter summarizes traditional steps and new strategies that can be performed by practitioners in the field to improve frozen semen quality and fertility.
Cryopreservation of Ejaculated Semen
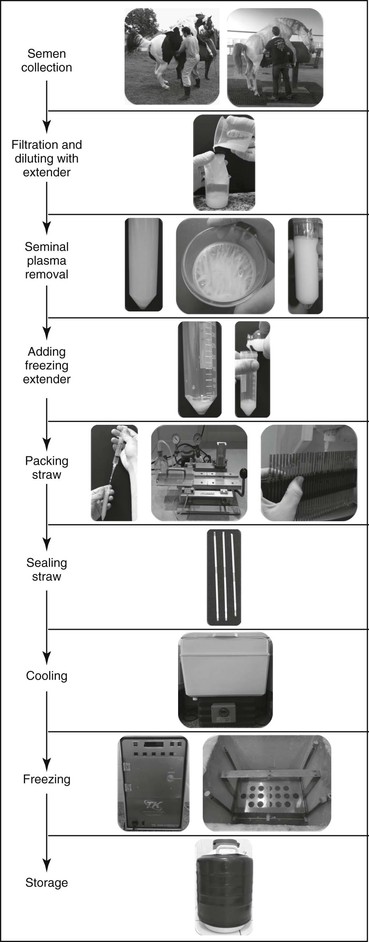
Preparing semen for cryopreservation involves collection, filtering with a nylon filter to remove dirt and the gel fraction from the ejaculate, concentrating the sperm cells, resuspending the sperm pellet in an extender with an added cryoprotectant, and packing the semen in straws. At this point, the semen is ready for cryopreservation, which must be performed according to the predefined freezing curves for the particular medium used. Following this step, the straw-packed sample can be stored in a nitrogen cylinder until thawed and used for insemination at the tip of the uterine horn (Figure 157-1).
Semen Collection
To collect semen from a stallion, the animal’s penis must first be cleansed to avoid contamination. For this purpose, the stallion must be aroused, and the penis washed with warm water.
Three methods are used to collect semen: with the stallion mounting a phantom, with the stallion mounting a properly restrained mare, or with the stallion in a standing position. The use of phantoms is considered the safest for both animal and operator, and collection is easy to perform, even with stallions accustomed to being collected while mounting a mare. When mares are used for semen collection, they must be in estrus and duly restrained with a twitch and breeding hobbles, and the tail should be wrapped to prevent injury to the penis. Collection with the stallion in the standing position is recommended for stallions that have difficulty mounting because of musculoskeletal problems. As the animal is teased by a restrained mare in estrus, the artificial vagina is slid onto the penis, and the stallion is left to perform the pelvic motions and ejaculate.
Of the models of artificial vaginas available, the Missouri and Colorado models are the most widely used in the United States, the Hannover is used most commonly in Europe, and the BotuCrio is the most frequently used in Brazil. These models are all based on the same design. Use of the disposable plastic inner liner is strongly recommended for sanitary reasons. Caution should be observed with the use of latex inner liners because they may be toxic to the sperm, especially when new.
To perform semen collection, the artificial vagina must be filled with water at 50° C (120° F), and may or may not be lubricated with a neutral lubricant gel. The authors do not use lubricant because most lubricants damage sperm.
If the animal has erection or ejaculation problems, chemical ejaculation may be used; several protocols for this procedure have been described in published literature. The reported rates of success with chemical ejaculation are varied, and the percentage of stallions that respond to this method of collection is low.
Concentrating the Sperm
To begin the cryopreservation process with stallion semen, the seminal plasma must be removed to concentrate the sperm. The plasma must also be removed because it can negatively affect sperm quality and viability.
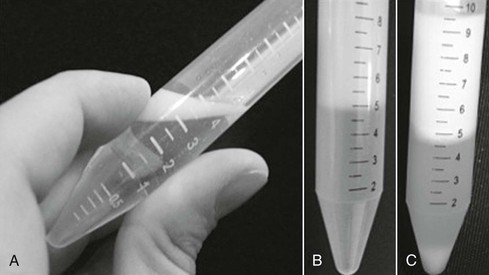
Several techniques are used for concentrating sperm from ejaculate. Centrifugation is most commonly used, although some studies have reported damaging effects of centrifugation on the sperm: the force and duration of centrifugation required to remove the seminal plasma can negatively affect sperm motility, integrity, and recovery rate (Figure 157-2).
To remove the seminal plasma with conventional centrifugation, a skim-milk–based extender is added to the semen in a 1 : 1 ratio before centrifugation at 600 × g for 10 minutes.
After centrifugation, the supernatant must be discarded by use of a catheter coupled with a syringe or vacuum pump and the pellet resuspended in the selected extender. If the resulting pellet is too compacted, the centrifugation force should be reduced in subsequent collections, or other techniques, such as filtering and cushioned centrifugation (see Chapter 155), should be used to minimize excessive packing of sperm.
Cushioned centrifugation is an alternative method for removing seminal plasma from the ejaculate while minimizing sperm damage. This method attempts to maximize sperm recovery from centrifuged equine semen by using high centrifugation forces while preventing damage to and packing of the sperm with a cushion placed at the bottom of the centrifuge tube. To remove the seminal plasma by means of cushioned centrifugation, the semen must be diluted 1 : 1 with skim-milk–based extender and stored in a 50-mL Falcon tube. A 1-mL cushion is carefully placed in the bottom of the tube with a catheter coupled to a syringe, and centrifugation is performed at 1000 × g for 20 minutes. After centrifugation, the supernatant must be carefully removed by aspiration with a catheter and syringe or other aspiration device. Next, the cushion must also be carefully removed with a catheter coupled with a syringe, and the pellet resuspended in the desired extender.
Another concentration method that could reduce sperm damage uses a filter made of a synthetic hydrophilic membrane1 with 2-µm pores that retain the sperm and allow passage only of the seminal plasma. The semen, diluted 1 : 1 with a skim-milk–based extender, is placed on the filter, which is gently touched against the inside of a 15-cm Petri dish. The pore size and capillarity of the filter are such that the seminal plasma flows through but the sperm are retained. A predetermined quantity of extender is then added to the filter, and the mixture is homogenized to resuspend the sperm. The entire process takes approximately 5 minutes, and the same filter may be used up to five times for the same stallion without affecting sperm quality and recovery.
Extender for Cryopreservation
The extenders for frozen equine semen are composed of substances to stabilize pH, neutralize the toxic products produced by sperm, protect against thermal shock, maintain electrolyte and osmotic balance, inhibit bacterial growth, and supply energy. The extenders must also contain cryoprotective substances to prevent formation of intracellular and extracellular ice. Several extenders are commercially available.2,3,4 Commonly used commercial extenders are summarized (Table 157-1).
Table 157-1
Summary of Commercially Available Extenders and Manufacturers
| Extender | Manufacturer |
| INRA Freeze | INRA, Nouzilly, France |
| Botucrio | Botupharma, Botucatu, Brazil |
| EquiPro CryoGuard | Minitube, Verona, United States |
| E-Z Freezin | Animal Reproduction Systems, Chino, United States |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree