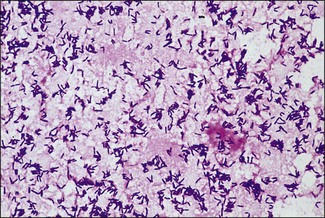
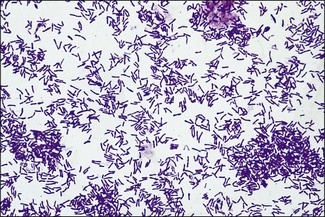
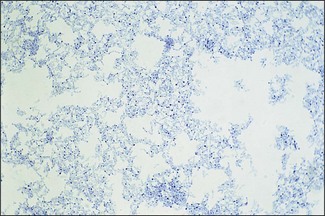
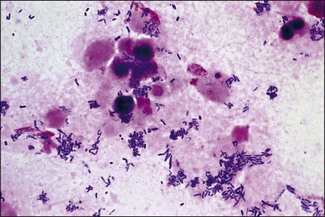
Chapter 9 The Corynebacterium species belong to the Corynebacteriaceae family of the Actinomycetales order. They are small, short, pleomorphic Gram-positive rods (about 0.5 µm in width) that occur in rod, coccoid and club-shaped forms. The corynebacteria are non-spore-forming, non-acid fast, catalase-positive, oxidase-negative and usually facultatively anaerobic. The animal pathogens are non-motile. Species of this genus infect humans and animals (Table 9.1). Stained smears from animal tissues often reveal groups of cells in parallel (‘palisades’) or cells at sharp angles to each other (‘Chinese letters’) (Fig. 9.1). Many have metachromatic granules (high-energy phosphate stores) and these are seen best in Corynebacterium diphtheriae (Fig. 9.2). Their nomenclature has undergone many changes over time: Table 9.1 Main diseases caused by the major pathogenic Corynebacterium species and Rhodococcus equi in veterinary medicine Figure 9.1 C. diphtheriae demonstrating club-shaped cells and ‘Chinese-letter’ patterns characteristic of the genus. (Gram stain, ×1000) Figure 9.2 C. diphtheriae with dark-staining (reddish-purple) metachromatic granules of polyphosphate. (Methylene blue stain, ×1000) Rhodococcus equi also belongs to the Corynebacteriaceae family of the Actinomycetales order. Rhodococcus species are aerobic and can appear as a Gram-positive coccus or as a rod. The bacterial cell is capsulated and sometimes weakly acid-fast. R. equi is catalase-positive and possesses an oxidative metabolism. This species is the only significant veterinary pathogen in the genus (Table 9.1) and is considered as an emerging human opportunistic pathogen in immunocompromised humans. Corynebacteria are found on the skin, mucous membranes (nasopharynx), and in the intestinal tracts of animals and humans. Corynebacterium pseudotuberculosis survived for one to eight days on inanimate surfaces and for seven to 55 days on particulate fomites such as wood shavings, hay, straw, and faeces (Augustine & Renshaw 1986). Lower temperatures generally extended the survival potential of this microorganism. The natural habitat of R. equi is soil, particularly soil contaminated with manure from horses and farm animals. Faecal contamination increases the rate of multiplication in soil because the volatile fatty acids in faecal material promote growth of the bacterium. The prevalence of R. equi pneumonia has been shown to be associated with the airborne burden of virulent R. equi (Muscatello et al. 2006). Lower soil moisture concentrations and lower pasture heights give rise to elevated airborne concentrations of virulent R. equi. Acidic soils may contribute to an increased proportion of virulent strains within an R. equi population (Muscatello et al. 2006). The main diseases caused by the major pathogenic Corynebacterium species and Rhodococcus equi in veterinary medicine are summarized in Table 9.1 and their virulence determinants are shown in Table 9.2. Table 9.2 Main virulence factors of pathogenic Corynebacterium spp. and Rhodococcus equi in veterinary medicine Corynebacteria are pyogenic bacteria causing a variety of suppurative conditions. Corynebacterium pseudotuberculosis is the aetiological agent of caseous lymphadenitis, a chronic contagious disease that affects goats and sheep, and can cause severe economic losses. It has also been recovered from other animal species (Table 9.1). It is a facultative intracellular bacterium which forms abscesses in the skin, lymph nodes (external or internal) and organs such as the spleen, lungs, liver, and kidneys. Transmission is usually via shearing, castration or any other skin wounds. Inhalation and ingestion are also possible ports of entry of the microorganism. Infected phagocytes disseminate the bacteria via the lymph or the blood to secondary sites where they cause lesions. The virulence of C. pseudotuberculosis is attributed to an exotoxin, phospholipase D (PLD) and to the surface lipid layer adjacent to the cell wall. The expression of fagB C D genes, which encode a putative iron uptake system, appears to contribute to virulence (Billington et al. 2002). Corynebacterium pseudotuberculosis can cause lymphadenitis and abscesses with granulomatous necrotizing lesions in humans and should be regarded as an occupational disease (Peel et al. 1997). Corynebacterium ulcerans is a rare cause of mild to severe (loss of the affected quarter) bovine mastitis. Virulence is attributed to the production of a PLD that genetically resembles the one produced by C. pseudotuberculosis. Corynebacterium ulcerans can also carry a phage that encodes for the diphtheria toxin. As a result, human diphtheria has been reported after consumption of raw milk (Barrett 1986). This pathogen is also occasionally associated with disease in cats and dogs and transmission from pets to humans can occur (Hogg et al. 2009). Corynebacterium bovis rarely causes clinical mastitis. In fact, it is considered to be a commensal of the bovine mammary gland and may protect the gland from infection with more virulent pathogens (Pociecha 1989). Corynebacterium kutscheri is a commensal of the upper respiratory and intestinal tracts of laboratory rodents. Infection is usually subclinical in mice and rats. However, when immunosuppressed, a pseudotuberculosis with high morbidity and mortality can appear in these animals. Corynebacterium renale, Corynebacterium cystitidis and Corynebacterium pilosum are commensals of the bovine reproductive tract. They are opportunistic pathogens causing simple or complicated ascending urinary tract infections (Table 9.1). C. renale is the most common cause of cystitis, ureteritis and pyelonephritis among the three species. C. pilosum is a milder pathogen often resulting in an uncomplicated cystitis. The virulence of these species is attributed to pili, which mediate adhesion to epithelial cells of the bladder, urease, which is responsible for hydrolysing urea and releasing nitrogen, and renalin (mainly in C. renale) that may play a role in lysis of host cells. R. equi is the cause of suppurative bronchopneumonia in foals. This disease increases in prevalence where high stocking rates occur. It is usually seen in foals of four to 12 weeks of age, possibly due to a decline in maternal antibody at about six weeks of age. The main route of infection is by inhalation. Heavily infected sputum may be swallowed by the affected foal leading to ulcerative colitis and mesenteric lymphadenitis. Infection has been seen in other species (Table 9.1). The bacterium can also multiply in soil enriched with equine faeces and may be a commensal in the intestine of horses. Rhodococcus equi is a facultative intracellular bacterium able to survive intracellularly through suppression of phagolysosomal fusion. The organism multiplies in and eventually destroys alveolar macrophages while neutrophils remain bactericidal. Rhodococcus equi has many virulence factors (Table 9.2) which probably play a part in the pathogenesis; however, the mechanisms involved are not all fully understood. In foals, R. equi virulence is associated with 80–90 kb plasmids, which include a pathogenicity island containing the virulence-associated protein (vap) gene family. In addition, nine chromosomal genes involved in fatty acid and lipid metabolism (choD, fadD13, fbpB), heme biosynthesis (hemE), iron utilization (mbtF), heat shock resistance and genes encoding chaperones (clpB, groEL), a sigma factor (sigK), and a transcriptional regulator (moxR) were significantly induced in R. equi cells growing inside macrophages (Rahman et al. 2005). In immunocompromized humans, R. equi necrotizing pneumonia is the commonest form of infection. However, extrapulmonary infections, such as wound infections and subcutaneous abscess, have also been described (Puthucheary et al. 2006). The corynebacteria are Gram-positive rods with varying degrees of pleomorphism (Fig. 9.3). R. equi is usually coccal but can be rod-shaped particularly in animal tissue (Fig. 9.4) and can be MZN-positive (weakly acid-fast). Figure 9.3 C. renale in a Gram-stained smear of bovine urine from a case of pyelonephritis. It shows extreme pleomorphism from club-shaped rods to coccal forms. (Gram stain, ×1000) For routine isolation sheep or ox blood agar is used with MacConkey agar to detect any Gram-negative contaminants that may be present. The plates are incubated at 35°C for 24 to 48 hours. A modified NANAT R. equi-selective agar medium has been used to recover R. equi from faecal samples (Grimm et al. 2007). Corynebacterium pseudotuberculosis produces small, white, dry colonies (Fig. 9.5). They can be surrounded by a narrow zone of haemolysis but often not until after 48 to 72 hours’ incubation (Fig. 9.6). After several days’ incubation the colonies can reach 3 mm in diameter and appear dry, crumbly and cream in colour. C. kutscheri produces small, whitish colonies that bear a resemblance to those of C. pseudotuberculosis (Fig. 9.7). Occasional strains are haemolytic. The colonies of C. bovis are small, white, dry, and non-haemolytic with a tendency to appear in the wells of plates inoculated with a milk sample as it is a lipophilic bacterium (Fig. 9.8). C. ulcerans produces slightly haemolytic, small, whitish colonies. Figure 9.5 C. pseudotuberculosis: small, white and dry colonies on sheep blood agar. Non-haemolytic at 24 hours’ incubation (see Fig. 9.6).
Corynebacterium species and Rhodococcus equi
Genus Characteristics
Previous name
Present name
Corynebacterium equi
Rhodococcus equi
Corynebacterium murium
C. kutscheri
Corynebacterium ovis
C. pseudotuberculosis
Actinomyces (Corynebacterium) pyogenes
Trueperella (Arcanobacterium) pyogenes (Chapter 10)
Eubacterium (Corynebacterium) suis
Actinobaculum suis (Chapter 10)
C. renale type I
C. renale
C. renale type II
C. pilosum
C. renale type III
C. cystitidis
Natural Habitat
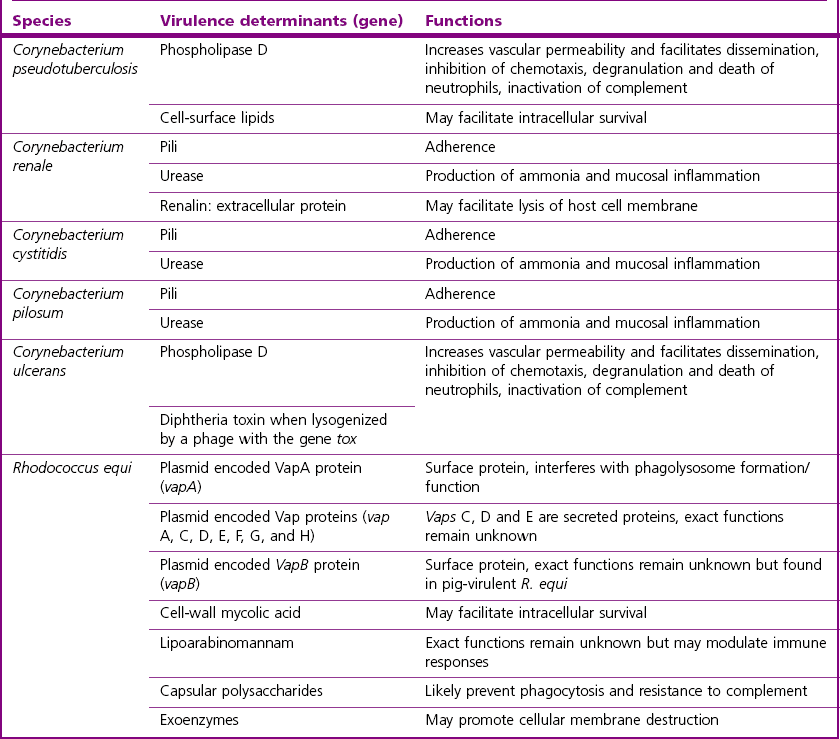
Pathogenesis and Pathogenicity
Laboratory Diagnosis
Direct microscopy
Isolation
Identification
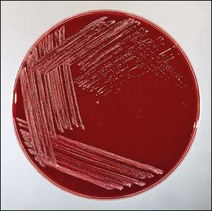
Colonial morphology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Corynebacterium species and Rhodococcus equi
Only gold members can continue reading. Log In or Register to continue