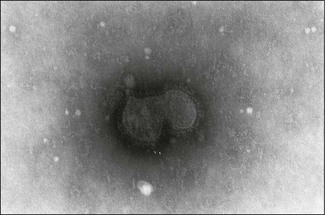
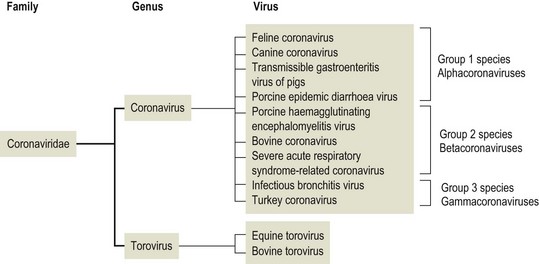
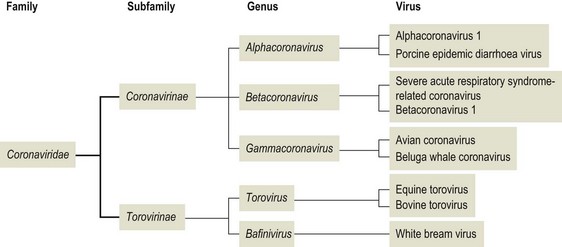
Chapter 62 The family Coronaviridae (Latin corona meaning crown) belongs to the Order Nidovirales, which also includes the family Arteriviridae. Coronaviruses are large, pleomorphic, enveloped viruses. Virions contain a single molecule of linear, positive-sense, single-stranded RNA. Coronaviruses have a crown-like appearance due to club-shaped glycoprotein peplomers projecting 15 to 20 nm from the envelope (Fig. 62.1). A peplomer is composed of a large trimeric viral glycoprotein (spike or S protein). These viral peplomers mediate viral attachment to specific cell receptors and fusion between the viral envelope and the cell membrane. The S protein is responsible for the induction of neutralizing antibodies during natural infection. Originally there were two genera in the family, Coronavirus and Torovirus. Coronaviruses have a helical nuclocapsid and are almost spherical with a diameter of 120–160 nm. Toroviruses have a tubular nucleocapsid, are approximately 130 nm × 40 nm and may appear disc-shaped, kidney-shaped or rod-shaped. Members of the genus Coronavirus form three genetic clusters or groups with distinct genetic and antigenic properties (Fig. 62.2). These clusters have formed the basis of additional genera in the family. The International Committee on Taxonomy of Viruses (ICTV) has divided the family into two subfamilies, Coronavirinae and Torovirinae, with the creation of new genera (Fig. 62.3), Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Bafinivirus. A number of viral species have been merged and renamed as follows; Alphacoronavirus 1 (comprising the viruses of feline coronavirus, canine coronavirus and transmissible gastroenteritis virus (TGEV) including the respiratory variant of TGEV, porcine respiratory coronavirus); Betacoronavirus 1 (comprising the viruses of human coronavirus OC43, bovine coronavirus, porcine haemagglutinating encephalomyelitis virus, equine coronavirus and the newly recognized canine respiratory coronavirus); Avian coronavirus (comprising the viruses of infectious bronchitis virus, turkey coronavirus, pheasant coronavirus, duck coronavirus, goose coronavirus and pigeon coronavirus). Coronaviruses display tropisms for respiratory and intestinal epithelium. Severe acute respiratory syndrome-related (SARS) coronavirus is a recently described member of the family, capable of producing serious disease in humans. There is evidence for an animal reservoir of SARS virus and Chinese horseshoe bats are suspected. The coronaviruses of veterinary importance are indicated in Table 62.1. Infections are usually mild or inapparent in mature animals but may be severe in young animals. Coronaviruses are aetiologically important in the common cold in humans. Feline coronavirus, canine coronavirus and transmissible gastroenteritis virus are closely related antigenically and genetically and are now referred to as alphacoronavirus 1. Evidence of torovirus infection has been found in horses, pigs, sheep, goats and cats (Muir et al. 1990). However, the clinical significance of these infections is questionable. Only bovine torovirus is recognized as capable of inducing enteric disease (Table 62.2). Table 62.1 Table 62.2 Feline infectious peritonitis, caused by certain strains of feline coronavirus (FCoV), is an invariably fatal, sporadic disease of domestic cats and other members of Felidae. It has a worldwide distribution and is a major problem in mutilple-cat households. Feline coronavirus is comprised of two closely related biotypes which vary in pathogenicity. The term feline enteric coronavirus (FECV) is used to describe strains that are present in virtually all multicat environments and cause little or no disease. The term feline infectious peritonitis virus (FIPV) is applied to those mutant strains of FECV responsible for cases of FIP (Vennema et al. 1998). Feline infectious peritonitis virus isolates display an enhanced ability to replicate in macrophages as a result of a mutation in the 3c gene (Pedersen 2009). Two serotypes of FCoV, distinguishable by neutralization tests, occur. Serotype 1 is the original FCoV, while serotype 2 is closely related to canine coronavirus and is probably a recombinant virus. Serotype 1 is the more prevalent serotype in Europe and North America, while serotype 2 is more prevalent in Japan. Both serotypes can cause clinically inapparent infections as well as FIP. Feline coronavirus serotype 1 strains are difficult to grow in cell culture, producing a slowly developing cytopathic effect. In contrast, serotype 2 strains grow more rapidly and give rise to a pronounced cytopathic effect. Cats shed FCoV in the faeces and oronasal secretions. Infection is acquired by kittens at a young age from their mothers or from other adult cats (Addie & Jarrett 1992). Feline infectious peritonitis is a sporadic disease occurring in individual cats in catteries or multicat households. Although cats of any age may be affected, disease is most commonly seen in young cats from six months to three years of age. It is thought that cats with FIPV do not generally transmit this mutant virus to other cats or at least only under certain circumstances. Infection with FCoV does not usually result in clinical disease. Factors considered important in the development of the disease include the age, immune status and genetic make-up of the host as well as the emergence of virulent virus strains (Addie et al. 1995). Infection with FECV is usually limited to enterocytes. In some animals a virulent FIPV strain capable of replication in macrophages and systemic invasion emerges. The development of effective cell-mediated immunity (CMI) may restrict viral replication and ultimately eliminate infection. When CMI is impaired or defective, virus replication continues leading to monocyte and perivascular macrophage activation, B cell activation and the production of non-neutralizing antibodies. The immune complexes formed from FIPV and the non-neutralizing antibodies are considered to be important in the development of immune-mediated vasculitis. However, the lesions are restricted to small- and medium-sized veins and there is evidence that the phlebitis is initiated by activated and FCoV-infected circulating monocytes (Kipar et al. 2005). The severity of this vasculitis influences the clinical presentation and the rate of progression of the disease. The incubation period ranges from weeks to months. The onset of clinical signs may be either sudden or slow and insidious. Early signs, which are generally non-specific, include anorexia, weight loss, listlessness and dehydration. Cats with the effusive form of the disease have fibrin-rich exudates in the abdominal or thoracic cavities. If the pleural effusion is marked, dyspnoea develops. The effusive form of the disease usually leads to death within eight weeks. In the non-effusive form of FIP, clinical findings are less characteristic. Signs associated with lesions in organs or tissues in the peritoneal cavity are present in about 50% of affected cats. Anterior uveitis, chorioretinitis and neurological signs may occur in up to 30% of cases. The course of the disease is usually protracted with animals surviving for several months. • Histological examination of affected tissues is the only procedure currently available for the definitive diagnosis of FIP. The demonstration of FCoV antigen in macrophages in the tissues by immunohistochemical means is confirmatory but obtaining appropriate tissue samples ante mortem is difficult. • Diagnosis of FIP in the live cat is usually based on history, clinical signs and a number of suggestive abnormal laboratory findings (Addie et al. 2004). • Pleural or peritoneal fluid from affected cats, which may contain fibrin strands, clots on standing and has a very high protein content with globulins comprising 50 to 82% (Sparkes et al. 1991). Cytological examination of the fluid typically reveals a predominance of macrophages and neutrophils consistent with the pyogranulomatous nature of the disease. Positive immunofluorescent staining of FcoV antigen in macrophages in the fluid is an excellent indication of FIP, but negative results can be obtained in cats that have FIP due to low numbers of macrophages. Rivalta’s test is an inexpensive way to distinguish transudates from exudates in cats. A reagent tube is three-quarters filled with distilled water to which is added one drop of acetic acid and thoroughly mixed. A drop of effusion fluid is carefully layered on the surface. A positive result is obtained if the drop retains its shape, stays attached to the surface or slowly moves to the bottom. The test is defined as negative if the drop disappears and the solution remains clear. A positive result has a predictive value of 86% in FIP cases (Hartmann 2005). • Haematological changes typical of the disease include neutrophilia, lymphopenia and, in chronic cases, a normocytic, normochromic anaemia. • Hypergammaglobulinaemia with a consequent serum hyperproteinaemia is frequently present. The albumin-to-globulin ratio is considered to be a better diagnostic indicator than total serum protein or gamma-globulin concentration. An albumin-to-globulin ratio of less than 0.8 is highly suggestive of FIP. Serum liver enzymes and total bilirubin may be raised in affected cats. High levels (>3 mg/mL) of the acute phase protein alpha one acid glycoprotein (AGB) are suggestive of FIP. • Diagnostic serological tests such as IFA and ELISA detect antibodies to FCoV. Titres must be interpreted with caution as many healthy cats have antibodies. However, they are of some value and considered indicative where high titres (>1/300) are detected in cats with clinical signs suggestive of FIP or in cats from a single-cat environment. Antibody titres by indirect immunofluorescence may be very high in some FIP cases but in other cases antibody titres have been found to be negligible (Sparkes et al. 1991). Serological testing is a valuable tool in identifying cats that are free of FCoV and for screening animals before introduction to a FCoV-free cattery. • Reverse transcription-PCRs for the detection of FCoV genome have been developed for evaluating blood and peritoneal/pleural effusions (Gamble et al. 1997, Kita et al. 2002) but are considered too sensitive, resulting in false positives (Addie et al. 2004). It has not been possible to design primers capable of distinguishing enteric FCoV from FIP-causing FCoV. The most promising ante mortem test is detection of FCoV messenger RNA within circulating monocytes using RT-PCR (Simons et al. 2005).
Coronaviridae
Virus
Host species
Disease
Feline coronavirus (FCoV)
Cats
Feline enteric coronavirus (FECV) is a strain of feline coronavirus which replicates in enterocytes. Relatively common cause of subclinical infection but may produce mild gastroenteritis in young kittens. Feline infectious peritonitis virus (FIPV) is a strain of feline coronovirus which initially replicates in enterocytes and subsequently in macrophages. Causes sporadic fatal disease of young cats typically presenting as an effusive peritonitis
Canine coronavirus
Dogs
Usually asymptomatic infection. Has also been isolated from dogs with diarrhoea; high morbidity but low mortality
Canine respiratory coronavirus
Dogs
Recently recognized as a cause of respiratory disease in kennelled dogs
Bovine coronavirus
Cattle
Important cause of diarrhoea in calves; associated with winter dysentery in adult cattle
Transmissible gastroenteritis virus (TGEV)
Pigs
Causes highly contagious infection. Infected piglets present with vomiting and diarrhoea; up to 100% mortality in newborn piglets. Porcine respiratory coronavirus, a deletion mutant of TGEV, induces partial immunity to the virulent virus
Porcine epidemic diarrhoea virus
Pigs
Enteric infection similar to TGE but with lower neonatal mortality
Porcine haemagglutinating encephalomyelitis virus
Pigs
Characterized by nervous disease or vomiting and emaciation (vomiting and wasting disease) in young pigs. Although infection is widespread, clinical disease is uncommon
Infectious bronchitis virus
Fowl
Causes acute, highly contagious respiratory infection in young birds and a drop in egg production in layers
Turkey coronavirus
Turkeys
Causes infectious enteritis (bluecomb disease)
Virus
Host species
Disease
Equine torovirus (Berne virus)
Horses
Originally isolated from rectal swab of a horse with diarrhoea in Berne, Switzerland. No clear association with disease
Bovine torovirus (Breda virus)
Cattle
Associated with diarrhoea in neonates, particularly if the animals have been deprived of colostrum
Feline infectious peritonitis
Pathogenesis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Coronaviridae
Only gold members can continue reading. Log In or Register to continue