CHAPTER 16
Controlled Substances
Mastery of the content in this chapter will enable the reader to:
• Define controlled substances.
• Identify drugs available for use in the veterinary practice.
• Explain the importance of logging all drugs used.
• Discuss the importance of managing controlled substances.
• Discuss methods used to inventory controlled substances.
• Describe methods used to order controlled substances for a veterinary practice.
• Identify appropriate documents to order Schedule II drugs.
• List the process used to report the loss of controlled substances.
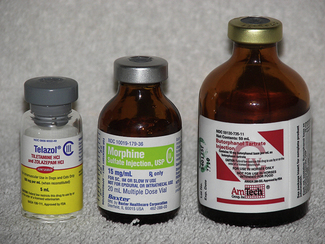
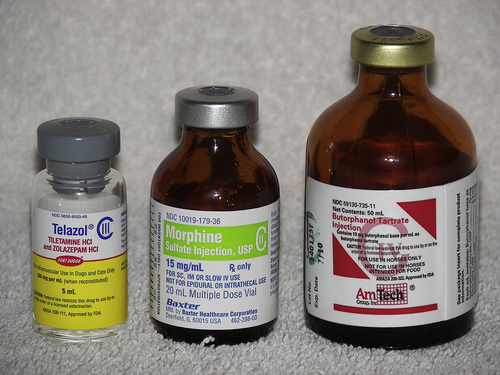
Controlled substances are drugs that have a high abuse potential and that must be regulated to help prevent those abuses (Figure 16-1). The Controlled Substance Act of 1970 was passed to reduce drug abuse by restricting certain substances with a high abuse potential. The act was established, and is controlled by, the Drug Enforcement Agency (DEA) and provides approved means for proper manufacturing, distribution, dispensing, and use through licensed handlers.
SCHEDULE OF DRUGS
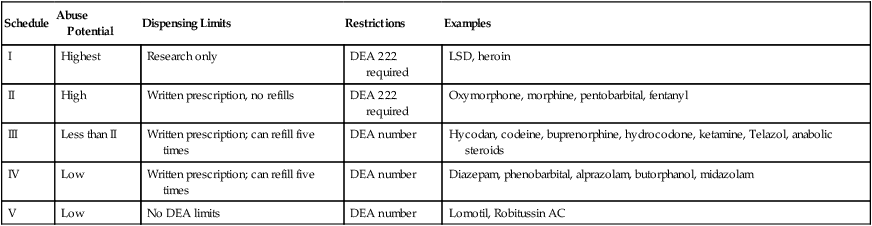
produce severe psychic and physical dependencies and include drugs such as morphine, oxymorphone, and pentobarbital. Table 16-1 lists drugs and their schedules.
Table 16-1
| Schedule | Abuse Potential | Dispensing Limits | Restrictions | Examples |
| I | Highest | Research only | DEA 222 required | LSD, heroin |
| II | High | Written prescription, no refills | DEA 222 required | Oxymorphone, morphine, pentobarbital, fentanyl |
| III | Less than II | Written prescription; can refill five times | DEA number | Hycodan, codeine, buprenorphine, hydrocodone, ketamine, Telazol, anabolic steroids |
| IV | Low | Written prescription; can refill five times | DEA number | Diazepam, phenobarbital, alprazolam, butorphanol, midazolam |
| V | Low | No DEA limits | DEA number | Lomotil, Robitussin AC |
MANAGEMENT OF CONTROLLED SUBSTANCES
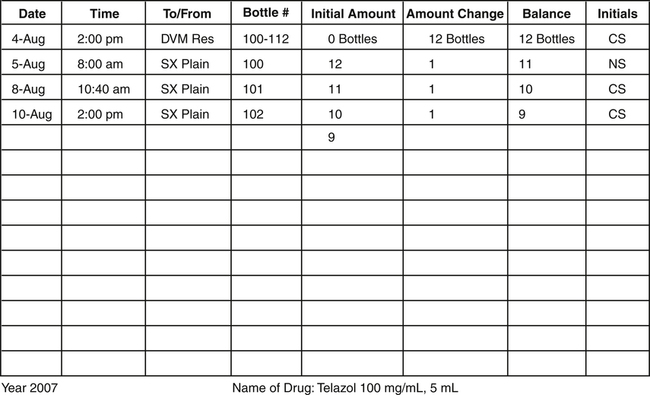
Figure 16-2 shows that 12 bottles of Telazol were received on August 4, 2000. Initially, there were no bottles available for use. Once the 12 bottles were added, the initial amount became 12 bottles. The bottles were assigned the numbers 100 through 112; these were the next numbers in sequence (99 bottles had been previously used). The stock supply list states that bottles were checked out to the surgical plain on August 5, 8, and 10 by two team members. Each time bottles are checked out, they must be recorded on this list and initialed by the team member removing the bottle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 PRACTICE POINT
PRACTICE POINT PRACTICE POINT
PRACTICE POINT