CHAPTER 35 Concurrent Disease Management
Hyperthyroidism and Chronic Kidney Disease,
Hyperthyroidism and Diabetes Mellitus,
Diabetes Mellitus and Obesity,
Diabetes Mellitus and Feline Lower Urinary Tract Disorders,
Heart Failure and Chronic Kidney Disease,
Management of Concurrent Pancreatitis and Inflammatory Bowel Disease,
Chronic Kidney Disease and Hypertension,
Immune Deficiency, Stress, and Infection,
Hyperthyroidism and Chronic Kidney Disease
Authors of a recent study reported that 14% of hyperthyroid cats had preexisting CKD on first assessment of thyroid disease.167 Whether there is a causal relationship between hyperthyroidism and CKD or whether both conditions simply occur in concert is not known because they are both common conditions in the older individual. The relationship between these two conditions is complex. It has been postulated that hyperthyroidism causes damage to the kidneys, which may contribute toward long-term development of CKD through several mechanisms. These include tubulointerstitial damage, ultimately fibrosis, and chronic interstitial nephritis caused by an increase in angiotensin II. In addition, systemic hypertension present in a proportion of hyperthyroid cats may contribute to renal damage through microinfarction and subsequent fibrosis. This last effect is recognized in the micropuncture studies on the remnant kidney cat model.157 Whether this occurs in hypertensive cats with naturally occurring CKD is unknown. However, it is also speculated that hyperthyroidism, in fact, merely masks a preexisting decline in renal function by increasing renal blood flow and hence glomerular filtration rate (GFR).
Diagnostic Challenges
The presence of CKD can make diagnosis of hyperthyroidism problematic because of suppression of thyroid hormones—the so-called sick euthyroid syndrome. Therefore in cats with clinical signs compatible with hyperthyroidism, a normal total thyroxine (total T4) should not be taken as evidence that the cat does not have this condition. Cats having both creatinine and total T4 concentrations in the upper normal reference range are likely to have both hyperthyroidism and CKD.238
If a normal T4 result is obtained in a cat suspected of being hyperthyroid, there are several options for further diagnosis. Repeating the same test on another occasion (for example, a few weeks later) can be helpful in those cases where the hyperthyroidism is mild, since fluctuation in levels of thyroxine can result in the total T4 being intermittently within the reference range. Measurement of free T4 by equilibrium dialysis is another option. This test is more sensitive in identifying hyperthyroid cats but carries a slightly higher risk of false-positive results (i.e., over diagnosing hyperthyroidism) so is not generally recommended as a screening test for hyperthyroidism.182 A total T4 greater than 30 nmol/L (greater than 2.33 µg/dL) in addition to a high free T4 is supportive of a diagnosis of hyperthyroidism; conversely, if the total T4 is less than 30 nmol/L (less than 2.33 µg/dL) the cat is extremely unlikely to have hyperthyroidism.238 Where available, analysis of endogenous thyroid-stimulating hormone (TSH) can be helpful, with hyperthyroid cats having low levels.238 Thyroid scintigraphy, triiodothyronine (T3) suppression, and thyrotropin-releasing hormone (TRH) stimulation tests226 can also be helpful in discriminating hyperthyroid from sick euthyroid patients. Unfortunately, all of these tests are affected by severity of concurrent disease, which can make interpretation of results difficult.
Assessment of urine specific gravity is usually helpful to define renal function, but this too can be affected by hyperthyroidism. A healthy urine specific gravity (USG) is generally considered to be greater than 1.040, with concentrations less than 1.035 taken as one of the indications of kidney disease. However, in hyperthyroid cats, the ability to produce concentrated urine is compromised, and a USG less than this figure can be obtained from cats that have healthy kidneys. Additionally, this healthy value varies with the type of diet fed. Cats fed strictly dry food should concentrate urine to greater than 1.045, whereas a USG of greater than 1.025 may be appropriate in cats fed exclusively canned/moist food.45
Mild proteinuria is a common finding in hyperthyroid cats.222 It is thought that the proteinuria may be present as a consequence of glomerular hypertension and hyperfiltration that is known to occur in the hyperthyroid state. The prognostic significance of proteinuria in hyperthyroidism is currently unknown, but it does tend to resolve with treatment, even in cats that develop azotemia.222,224
Both hyperthyroidism and CKD are conditions associated with a higher incidence of urinary tract infections. For example, one study showed that 12% of hyperthyroid cats suffered from urinary tract infections.158 Unfortunately, most of these cats suffer from clinically silent urinary infections and may only show vague clinical signs, such as weight loss and lethargy. It is important to be suspicious of infection and culture the urine to identify and treat any of these additional problems, because it will benefit the cat’s current and long-term prognosis.
Treating Cats with Concurrent Hyperthyroidism and Chronic Kidney Disease
All treatments for hyperthyroidism have the potential to worsen kidney function.58 This is because the hyperthyroid condition increases renal blood flow and GFR. When the hyperthyroidism is treated, the increased cardiac output and renal blood flow to the kidneys decreases. This results in a decrease in GFR by up to 50% of the pretreatment level.11,16,83 For many hyperthyroid cats, this return to normality is not associated with kidney problems. However, in a proportion of patients, this reduction in blood flow has the potential to unmask kidney disease that was not previously recognized, allowing the preexisting kidney disease to manifest itself clinically. In those cats in which renal disease has been documented before treatment is started, treatment has the potential to worsen renal function and may precipitate a crisis. The reported frequency of this complication has varied in publications. In one report, one third of patients developed this complication following treatment with radioiodine, while other reports have found even higher numbers of cats experiencing a crisis after treatment.58,212 Affected cats become azotemic and may start to show clinical signs of renal disease. Significant decreases in renal function are generally evident by 4 weeks posttreatment, after which time GFR stabilizes with very little deterioration, depending on the degree or stage of renal disease.16,233,234
A number of studies have shown that it is not possible to predict accurately which patients will reveal CKD following treatment of hyperthyroidism. These studies have evaluated pretreatment laboratory variables, such as serum biochemistry, USG, proteinuria, and hematology.16,192,222,234 In general, no significant differences have been seen when comparing the pretreatment parameters in cats that did develop a posttreatment renal azotemia with those that did not. Although USG is reduced in cats with CKD, it also can be reduced as a consequence of hyperthyroidism, so analysis of this parameter alone is not sufficient to be helpful. Equally, although a pretreatment USG of greater than 1.035 is often reassuring, it cannot be viewed as a guarantee that the cat will not develop a posttreatment renal azotemia.192 The fact that some cats with primary renal azotemia remain able to concentrate urine to 1.045 or greater complicates the interpretation further.174
GFR is reported to be of some value in predicting posttreatment renal azotemia.2,234 One study reported some value in using a combination of pretreatment GFR, USG, and total T4 in predicting which patients developed a posttreatment renal azotemia.234 In the same study, there was a significant difference in pretreatment GFR and USG in those cats that eventually developed posttreatment renal azotemia. Unfortunately, assessment of GFR is not readily available in clinical practice, limiting its usefulness. Iohexol clearance has been evaluated and could be a clinically feasible tool; however, it has not become part of mainstream diagnostics.11 The usefulness of other markers of renal function, such as the NAG index (urinary N-acetyl-beta-D-glucosaminidase to urinary creatinine ratio) and urinary retinol binding protein, are still being evaluated.144,232
If a cat is known to have CKD before medical treatment for hyperthyroidism is started, it is probably advisable to start treatment with a lower dose of medication initially while monitoring the renal values closely. For example, if using methimazole, a starting dose of 1.25 to 5 mg every 24 hours should be considered. If any problems are seen, then the methimazole dose can be lowered or the treatment may be discontinued. If, conversely, medication is not associated with renal or other adverse effects, the dose can be titrated to induce and maintain euthyroidism. Frequent and regular re-evaluations are essential to facilitate optimal management of both CKD and hyperthyroidism. The author recommends initial assessment of renal parameters at 3 and 6 weeks following the start of treatment.39
Ongoing management of patients with both CKD and hyperthyroidism requires attention to both conditions. International Renal Interest Society (IRIS) guidelines (http://www.iris-kidney.com) should be followed with respect to staging and management of CKD and any complications present as a result of this. Where possible, attempts should be made to induce and maintain euthyroidism as discussed above. In those patients where euthyroidism is associated with a clinical and biochemical worsening of renal disease, it may be necessary to titrate therapy to achieve the best balance possible. The individual priorities of each patient need to be considered to determine which therapy and approach is most appropriate. For example, in some patients, suboptimal control of the hyperthyroidism may be tolerated clinically, whereas euthyroidism may be associated with severe renal dysfunction and a clinical crisis. Success of treatment should be gauged on clinical response to treatment as well as assessment of laboratory parameters. Accurate assessment of body weight and body condition score is vital in addition to a thorough history, general clinical examination, and blood pressure measurement. Fortunately, in many cats, it is possible to achieve a balance between these two conditions and gain a good treatment outcome.
Hyperthyroidism and Diabetes Mellitus
Prevalence
Hyperthyroidism in cats was virtually unknown until the late 1970s and is now the most common endocrine disease of cats and one of the most common diseases in older cats. Diabetes mellitus (DM) is also a commonly encountered endocrine problem in older cats, and its prevalence also has increased dramatically in the last three decades because of a large increase in the prevalence of obesity. According to Joslin (writing in 1934),128 in people, “the subject of hyperthyroidism and diabetes, as a combination of diseases, is such a small one that it permits but little to be said about,” even though diabetes not infrequently co-exists with hypothyroidism and hyperthyroidism in human patients. It is not known how frequently the two diseases co-exist in cats, but anecdotally, it is well known that they can occur concomitantly. There are, however, no epidemiologic or pathophysiologic data linking hyperthyroidism with diabetes, nor is an increase in fasting blood glucose a common feature of the hyperthyroid cat.
Patient Signalment and Risk Factors
Although the increase in both diseases has occurred during the same time span, that is, the last 3 decades, it does not appear that identical factors are involved in the pathogenesis except for the fact that hyperthyroidism and DM occur primarily in older cats, and most patients are more than 10 years old. Hyperthyroidism and DM in young cats are extremely rare, with the exception of DM in Burmese cats in Australia. Other risk factors for DM are body condition (obesity), sex, and reproductive status.205 At highest risk would be an old, obese, neutered male cat. A genetic predisposition appears to occur in Australian Burmese cats. The marked increase in DM in cats can be traced to the increase in feline obesity.
Several investigators from different continents have evaluated the risk factors for hyperthyroidism and have presented very similar results despite their geographic differences.64,173,237 As stated, older domestic short- and long-haired cats were more likely to develop the disease than young or purebred cats. The risk increased with increasing age. There was no difference in the risk for males versus females in one study, whereas female cats were identified at higher risk in the others. Hyperthyroid cats were more likely to have used a litter box, to be fed wet cat food from a pop-top can, or were fed all categories of table food, including high-fat dairy products. The plasticizer compounds bisphenol A and phthalates have been suspected, without proof, as a causative agent for obesity and diabetes in man and for hyperthyroidism in cats, but no linkage has been ascertained to date. Environmental factors appear to play an important role, because hyperthyroid cats were more likely to have been exposed to smokers in their environment and to household insecticide treatments. Other risk factors include sleeping on the floor, exposure to organic fertilizers, and dental disease. Interestingly, hyperthyroidism was less likely in multicat households compared with single-cat households.
Clinical Signs
Less predictably, both conditions may present with muscle weakness, vomiting, and diarrhea. In addition, cats with extreme hyperthyroidism may show agitation. The diabetic cat with acromegaly may gain, rather than lose weight.15
Pathophysiology
Hyperthyroidism and diabetes are both catabolic states. Hyperthyroidism is caused by excessive secretion of thyroid hormones (triiodothyronine [T3] and thyroxine [T4]) by hyperplastic or adenomatous thyroid glands, rarely by malignant thyroid carcinomas.106,181 Hyperthyroidism in cats is most similar to hyperthyroidism in people caused by toxic nodular goiter. Abnormalities of the G protein and 3′-5′-cyclic adenosine monophosphate (cAMP)-signaling pathway have been implicated in the pathogenesis in both species.95,178 Although in people antibodies against islet antigens are found with autoimmune thyroid disease, there is no evidence that a similar connection exists in hyperthyroid cats. Autoimmune processes have not been shown to play a role in either disease, and islet antigens have not been detected in diabetic cats.110
Thyroid hormones (TH) affect many aspects of metabolism and energy homeostasis, and in general terms, can be viewed as antagonists to insulin. The metabolic alterations during thyrotoxicosis represent direct effects of TH on the expression of TH-responsive genes and are mediated by binding of T3 to TH receptors in peripheral organs.10,253 Thyrotoxic human patients exhibit insulin resistance, that is, the effectiveness of insulin in muscle, fat, and liver is hampered.15,128,136,178,179 Because one of the main roles of insulin is the control of glucose homeostasis, increased insulin resistance is seen as a decrease in glucose tolerance. In a study comparing healthy with hyperthyroid cats, glucose clearance was decreased in hyperthyroid cats, and insulin secretion was increased during an intravenous glucose tolerance test.105 This pattern is characteristic for peripheral insulin resistance, which causes decreased uptake of glucose into muscle and fat tissue. However, fasting blood glucose concentrations were still normal, suggesting that hepatic glucose output was still normal.
It is known that hyperthyroidism by itself causes increased hepatic glucose production and a dramatic increase in Krebs cycle flux.59,131,137 One might therefore expect excess glucose production in thyrotoxicosis. However, we have shown in cats that pyruvate cycling flux, a futile cycle, is also stimulated by thyroid hormone, thereby negating an effect on gluconeogenesis.136 It is conceivable that in hyperthyroid cats, gluconeogenesis is kept low, and fasting blood glucose is kept in the normal range through enhancement of this futile cycle. It has been shown in hyperthyroid rats that gluconeogenesis was only increased 20%, because pyruvate cycling decreased gluconeogenesis by more than two thirds compared with what would be seen were pyruvate cycling not operative, suggesting that pyruvate cycling plays a functional role in protecting against overproduction of glucose in liver.126 Hyperglycemia is therefore not a feature of hyperthyroidism. Contrary to popular belief, there is no evidence that hyperthyroidism increases intestinal glucose absorption in cats, and data from other species are highly controversial.165,189
Diagnosis
Serum Total Thyroxine (TT4) Concentration
It has been well documented that nonthyroidal diseases suppress circulating TT4 concentrations in cats. In fact, diabetes mellitus has one of the most profound effects to reduce TT4.182 If TT4 is normal in a diabetic cat suspected of hyperthyroidism, other tests are indicated, such as circulating free T4 concentration by dialysis (FT4D), nuclear scintigraphy, T3 suppression test, or thyrotropin-releasing hormone (TRH) stimulation. However, normal, and occasionally high, TT4 and high free T4 has also been described in obese cats as well (see below). The functional tests (TRH stimulation, T3 suppression, scintigraphy) have not been systematically studied in obesity, but none of these tests have shown high diagnostic specificity in cases of hyperthyroidism with concomitant nonthyroidal illness.180,182,226
Fructosamine
In unregulated diabetic cats, serum fructosamine concentrations are high, whereas in hyperthyroid cats, concentration of serum fructosamine may be low because of accelerated protein turnover.191 Therefore fructosamine concentrations should not be used solely as an indicator of glycemic control in the diabetic cat with concurrent hyperthyroidism.
Diabetes Mellitus and Obesity
Obesity is the most common nutritional disorder, and diabetes mellitus (DM) is one of the most common endocrine diseases in cats. The prevalence for both has increased dramatically in the last 3 decades. Obesity is now thought to occur in about 40% and DM in about 0.5% to 1% of the cat population. Environmental factors, such as unrestricted food intake and reduced physical activity, are largely responsible for the modern epidemic of obesity. Obesity and DM are tightly linked to each other in cats, as they are in people. It is thought that feline obesity increases the risk to develop diabetes threefold to fivefold. Other risk factors for diabetes are gonadectomy and sex. Obese male neutered cats have the highest risk to develop the disease.205
Definition of Diabetes Mellitus
Unlike in people, a diagnosis of DM in cats is usually only made when the animal exhibits obvious clinical signs of hyperglycemia. In people, an oral glucose tolerance test (OGTT) is a frequently used test to document diabetes but is rarely applied in pets. In asymptomatic people, a diagnosis of impaired glucose tolerance or diabetes is usually made based on fasting glucose concentrations and the response to a 75-g glucose load. Strict criteria have been established for the interpretation of the results to separate healthy people from people at risk of developing diabetes or already having diabetes. Oral glucose tolerance testing has recently been described in cats.108 However, as in dogs, the OGTT in cats is associated with high variability of results and is not recommended as a routine clinical diagnostic test. The intravenous glucose tolerance test, although associated with less variability, is labor intensive and not suited for use in clinical practice. Therefore early recognition of cats at risk of developing diabetes is difficult, and no clear pattern of easily measurable parameters has emerged yet that would indicate development or progression of the disease process.
Definition of Obesity
Obesity occurs when energy intake exceeds energy output. There are subjective as well as objective methods to measure increases in body mass. One of two (5- or 9-point scales) condition scoring systems are frequently used in practice.142 Although subjective, they can be easily performed by one person. Longitudinal assessment (i.e., repeated over time) of animals should preferably be performed by the same person to decrease the variability of results. A score of 3/5 or 5/9 indicates that the cat is well proportioned, that is, of normal weight, while a body condition score of 5/5 or 9/9 indicates that the cat is obese and has heavy deposits of fat. Values in between indicate the increase in fat deposits as the numbers rise. Girth circumference may be measured behind the last rib and is a good objective indicator of obesity. Similar to body condition scoring, it can also be performed by one person. Its results correlate very well with fat measurements using more sophisticated methods, such as dual-energy x-ray absorptiometry (DEXA).109 Because normal girth values are not available for different cat breeds, at this time, this method should only be used to follow the body condition in a given individual animal over time. Plain radiographs may also be helpful in assessing condition by evaluating falciform and paralumbar fat deposits. Other objective methods, such as body mass index, DEXA scanning, or magnetic resonance imaging have also been performed in cats, but not usually in clinical practice. Because normal ranges for those techniques are not available for different breeds, these tests are also valuable only when performed over time in the same cat.
The Link Between Obesity and Diabetes
It is known that obese cats are insulin resistant. Insulin resistance is the condition in which normal amounts of insulin do not produce a normal insulin response from cells. Insulin resistance is usually measured with a method called the euglycemic hyperinsulinemic clamp. Simply explained, this is a technique in which a constant amount of insulin is infused. Glucose is also infused in the amount necessary to keep blood glucose concentrations in the euglycemic range. The more sensitive a cat is to the effect of insulin, the more glucose needs to be infused. It has been shown in cats that every kilogram in weight gain leads to a 30% loss in the sensitivity to insulin.111,112 Insulin resistance is seen in muscle, fat, and liver in obese people. In obese cats, the response to insulin is tissue dependent, and even in long-term obese cats, insulin resistance is only seen in adipose and fat tissue. In muscle and fat, the following changes are seen with obesity-induced insulin resistance:
• The expression of the insulin-sensitive, high-Km (i.e., requires a large amount of glucose to achieve maximum reaction velocity) glucose transporter type 4 (GLUT4) is decreased.
• The expression of GLUT1, the insulin-insensitive low-Km (i.e., requires only a small amount of substrate to become saturated and reach maximum velocity) glucose transporter type 1, is unchanged.
This leads to a decrease in glucose clearance when cats are challenged with a high glucose load. These cats do not show a change in glycosylated hemoglobin, and baseline glucose concentrations remain normal even in the long-term obese cat.20
An interesting phenomenon, and one that explains the normal baseline blood glucose in obese cats, is the fact that the liver remains responsive to insulin. In a recent study, using nuclear magnetic resonance spectroscopy, we were able to show that insulin suppresses hepatic endogenous glucose production (EGP) by reducing glycogenolysis and gluconeogenesis in obese cats. These cats were hyperinsulinemic, indicated by approximately doubled baseline peripheral insulin concentrations and yet had no difference in glucose concentrations compared with lean cats. This suggests that hepatic autoregulation is intact in obese cats despite peripheral insulin resistance and impaired glucose clearance.136 The decreased EGP in obese cats might be a compensatory mechanism to ensure normal fasting glucose concentration. It appears therefore that a loss of hepatic autoregulation is an important step in the pathogenesis of diabetes in cats.
Obesity and diabetes are characterized by quantitative and qualitative alterations of insulin secretion. Insulin normally is secreted from beta cells in a biphasic manner in response to high glucose during an intravenous glucose tolerance test. In the cat as in people, other fuels such as amino acids potentiate the secretion of insulin in the presence of glucose. Characteristic changes that are seen in obese cats are a marked increase in the second or maintenance phase of insulin release. The amount of insulin secreted during the second phase is primarily an indicator of glucose uptake into peripheral tissues. Because of the change in glucose transport and the delayed clearance of glucose in obese insulin resistant cats, insulin secretion is increased to overcome the resistance. Over time, the persistent oversecretion leads to a decrease in total insulin secretory capacity, and the animal becomes diabetic when insulin no longer leads to a normal response.60,107 It is not known when in the course of the development of diabetes hepatic insulin resistance develops and when increased hepatic glucose output contributes to increasing glucose concentrations (known as glucose toxicity) even in the basal state, thereby worsening the demand on beta cells and accelerating their exhaustion.114 Eventually, beta cells will undergo apoptosis (programmed cell death).60,195
Islet amyloid is one of the characteristic features of feline diabetes and of human type 2 diabetes and is associated with a loss of beta cells. The precursor protein of islet amyloid is the hormone islet amyloid polypeptide (IAPP), which is co-localized in secretory granules of beta cells with insulin and is co-secreted with insulin.129 It is not known why amyloid is formed in the diabetic cat. It is currently believed that the loss of beta cells associated with islet amyloidosis is actually caused by membrane permeable cytotoxic oligomers of IAPP rather than the final product, amyloid, and protective mechanisms in beta cells keeping IAPP in a soluble form must fail to allow these cytotoxic products to form.93 Because the endoplasmic reticulum is responsible for the proper folding of proteins, including amyloid, it appears that any process that disturbs its function will lead to oligomer formation (Figure 35-1). There are no data about the occurrence of islet amyloid in obese cats compared with age-matched lean cats; however, we have shown that obese cats hypersecrete IAPP as well as insulin, which, long-term, might lead to disruption of regular secretory pathways.
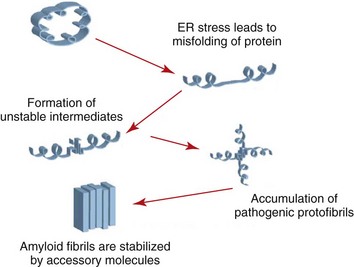
FIGURE 35-1 The effect of stress on endoplasmic reticulum amyloid formation.
(From Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR: Type 2 Diabetes mellitus as a conformational disease, JOP 6:287, 2005.)
Other hormones likely play a role in the progression from obesity to diabetes in cats (Table 35-1). Leptin is a hormone that is secreted from adipocytes. In lean animals, it leads to satiety and increases energy expenditure. It is known that obese cats have leptin resistance. Similar to the definition for insulin resistance, leptin resistance is the condition where normal amounts of leptin do not cause a normal response. Leptin concentrations are high in obese cats; yet, there is no decrease in food intake or increase in energy expenditure as would be expected in a cat that would respond normally.6,112 The concentration of adiponectin, a hormone secreted from adipose tissue, which modulates glucose and lipid metabolism, is low in obese cats and correlates with insulin sensitivity.112
TABLE 35-1 Hormonal and Metabolic Changes in Obese Cats Compared with Lean Cats
| Hormone | Concentration in Obese Cats | Effect |
|---|---|---|
| Adiponectin | Low | Decreased glucose and fat metabolism |
| Glucagon-like peptide-1 | Low | Decreased hepatic glucose production |
| Decreased gastric emptying | ||
| Decreased satiety? | ||
| Lower glucose-dependent insulin release | ||
| Insulin | High insulin resistance | Exhaustion of beta cells |
| Leptin | High leptin resistance | Decreased energy expenditure |
| Decreased satiety? | ||
| Thyroid hormone | Higher but usually still within the normal range—thyroid hormone resistance? | Decreased energy expenditure |
Thyroid hormone changes contribute to the metabolic alterations of obesity. The energy expenditure is lower in obese cats than lean cats but increases with administration of triiodothyronine (T3), indicating that thyroid hormone is involved in the low heat production. It has also been shown that free T4 has the strongest positive correlation with indices of obesity and increases with increases in body mass index, girth, leptin, and fatty acids. This suggests that obesity leads to a form of thyroid hormone resistance.103,104
Glucagon-like peptide 1 (GLP-1) has recently been examined in obese cats and was lower than in lean control cats.108 Changes in GLP-1 concentrations have been associated with changes in glucose control in obese people and people with diabetes. It increases insulin secretion through activation of GLP-1 receptors on beta cells and increases the transcription of the proinsulin gene. It also inhibits glucagon secretion and regulates glucose homeostasis through decreasing glucose production by the liver, decreasing the rate of gastric emptying, and decreasing central effects on satiety.
Obese cats show a marked change in lipid metabolism. We found that obesity does have a significant effect on both plasma lipids and lipoprotein concentrations. Plasma triglycerides were found to be increased with obesity as was the very-low-density lipoprotein fraction (VLDL), the main carrier of triglycerides, whereas the low-density lipoprotein fraction (LDL) was unchanged, and the high-density lipoprotein (HDL) fraction was decreased in long-term but not short-term obese cats compared with lean cats. Using nuclear magnetic resonance spectroscopy, we measured particle size and concentration within each of the lipoprotein fractions and found that obese cats have very similar changes to obese people who are at risk of developing atherosclerosis and cardiovascular disease. Those changes include more large VLDL particles, more medium- and small-sized LDL particles, and more small HDL particles.127 Despite these changes, atherosclerosis and cardiovascular disease have not been described in obese cats, and only one study has suggested that there might be an increase in cardiovascular disease in diabetic cats; however, this needs to be confirmed in a well-controlled study. Not surprisingly, obese cats have a lower expression of peroxisome proliferator-activated receptors (PPARs), which are transcription factors that play a major role in the regulation of lipid, carbohydrate, and protein metabolism.104
Both high plasma glucose and fatty acid concentrations are involved in the mediation of oxidative stress through the generation of reactive oxygen species (primarily oxygen and nitrogen). Oxidative stress may play a role in the progression from obesity to diabetes, but so far this has not been studied in cats. Antibodies to islet antigens do not seem to play a role in the pathogenesis of diabetes in cats.110 Interestingly, the metabolic sequelae of obesity are not at all, or only to a very limited degree, influenced by dietary components.
In conclusion, obesity is a major risk factor for the development of diabetes. It appears that loss of hepatic autoregulation may be the switch between obesity and diabetes in cats. Once the animal shows hyperglycemia, the toxic effects of glucose become evident as already shown in cats in 194860 and, in beta cells, lead to exaggerated apoptosis and further loss of beta-cell mass. It is important to know that the changes described above for obese cats can be reversed with a simple treatment, weight loss. This is one more reason to make sure feline obesity is recorded and treated before the path to diabetes becomes a one-way street.
Diabetes Mellitus and Feline Lower Urinary Tract Disorders
Etiology and Prevalence
Diabetes mellitus is one of the most common feline endocrine diseases. The etiology of type 2 DM is multifactorial, with obesity, genetics, diet, and islet amyloidosis involved in the development of this form of DM in humans and cats.172,190,255 FLUTD occurs in approximately 1% to 3% of cats seen at general veterinary practices; the etiology is multifactorial, and a cat may be predisposed to the condition because of genetic, dietary, and environmental factors.31,32,34,118,149
Patient Signalment and Risk Factors
Risk factors for the development of diabetes mellitus in cats include increased body weight (>6.8 kg), older age (>10 years) and neutering.176,185 Neutered males (NM) are 1.5 times more likely than females to develop diabetes mellitus. FLUTD is seen most commonly in cats that are young to middle aged, overweight, kept indoors, fed dry food ad libitum and live in a multianimal household.31,32,34,38 Neutered cats are more susceptible, and the risk of urinary tract obstruction is greatest in males.149 Concurrent FLUTD and DM is most likely to occur in obese middle-aged NM cats kept indoors.
Clinical Signs
Obesity combined with fasting or postprandial hyperglycemia may be the only clinical sign of early type 2 DM. Owners of diabetic cats may report gait abnormalities, weakness, inappropriate elimination (particularly if the litter box is large or placed in a remote location), and problems with jumping prior to the onset of polydipsia and polyuria.85 Late signs of DM in cats include polydipsia, polyuria, anorexia, lethargy, and weight loss. The most common physical examination findings in cats suffering from DM are lethargy and depression (70%), dehydration (63%), unkempt hair coat (35%), and muscle wasting (47%).85,185 Plantigrade rear limb stance resulting from diabetic neuropathy is observed in approximately 8% of diabetic cats.185
Cats with lower urinary tract disease can present with signs of dysuria, hematuria, pollakiuria, inappropriate urination, and/or urethral obstruction.91,118 Nonobstructive cases are self-limiting, usually resolving within 5 to 10 days.
Pathophysiology
To summarize the current hypothesis of the pathogenesis of type 2 DM, peripheral insulin resistance (resulting from obesity, elevated plasma islet amyloid polypeptide [IAPP], or both) causes chronic stimulation of insulin production in the pancreatic beta cells.190 Amylin is co-synthesized with insulin; therefore abnormal insulin secretion causes IAPP to accumulate in the beta cells.172 The high local concentration of IAPP causes polymerization of IAPP to form insular amyloid. Eventually a vicious cycle of increased amyloid production and chronic hyperglycemia leads to beta-cell failure and apoptosis (programmed cell death).
Stress plays an important role in the pathogenesis of feline idiopathic cystitis. Recent studies indicate that FIC, the most common cause of FLUTD, may be caused by placing a “susceptible” cat into a “provocative” environment.”244,246,247,249 Activation of an abnormal pituitary-adrenal axis caused by genetic or epigenetic abnormalities, coupled with catecholamine excess caused by environmental stressors, leads to local bladder inflammation. Most cats with signs of FLUTD are obese, and the role of obesity in these diseases is poorly defined. Increased visceral fat causes inflammation46,166 and predisposes to diseases, such as type 2 DM in both cats and humans. In fact, in the author’s experience, many cats that eventually develop DM have had episodes of FLUTD prior to presentation with signs of diabetes.
Diagnosis
Common clinicopathologic features of diabetes mellitus in cats include fasting hyperglycemia, hypercholesterolemia, increased liver enzymes (ALP, ALT), neutrophilic leukocytosis, possible proteinuria, variable urine specific gravity, bacteriuria, and glucosuria.48 Many cats are susceptible to stress-induced hyperglycemia. In addition, renal glucosuria may be found in animals with renal tubular disease and with stress-induced hyperglycemia. Serum fructosamine evaluation may be beneficial in differentiating early or subclinical diabetes mellitus from stress-induced hyperglycemia in the cat. Serum fructosamine is formed by glycosylation of serum protein, such as albumin, and the concentration of fructosamine in serum is directly related to blood glucose concentration. One study of 17 normal cats showed that transient glucose administration (1 g/kg 50% glucose solution, intravenously) did not cause increased serum fructosamine concentrations.153
Environmental and Dietary Therapy
A lower-carbohydrate, higher-protein diet may ameliorate some of the abnormalities associated with diabetes mellitus in the cat. Initial studies using a canned high-protein/low-carbohydrate diet and the starch blocker acarbose have shown that 58% of cats discontinue insulin injections, and those with continued insulin requirements could be regulated on a much lower dosage (1 to 2 U every 12 hours).159 Comparison of canned high-fiber versus low-carbohydrate diets showed that cats fed low-carbohydrate diets were 3 to 4 times more likely to discontinue insulin injections.13 The diet formulation is critical in that most dry cat food formulations contain excessive carbohydrates; therefore canned cat foods and preferably higher protein foods should be used for initial treatment of diabetic cats. Caution should be used when initially changing from dry to canned foods as insulin requirements may decrease and a reduction in insulin dosage may be required.
Recent evidence suggests that signs of FIC can be reduced by the use of a multimodal environmental modification program.33 An appropriate number and positioning of the litter boxes should allow the cats to have free access. Although covered litter boxes may be thought to provide a safe and private place to eliminate, many cats will not use them. Daily scooping of urine and feces is essential, and full cleaning of the box and replacement of the litter should take place at least once a week. This is particularly important in diabetic cats with FLUTD because of the presence of polyuria as well as possible odors. The use of Feliway (Ceva Animal Health, Buckinghamshire, England) diffusers during this process is recommended, because it has been shown to reduce signs of defensive aggression and passive withdrawal.90
Drug Therapy
Cats with diabetes mellitus should be treated with diet and insulin or possibly diet and oral hypoglycemic agents71,190; however, the presence of concurrent DM and FLUTD often requires intensive control of hyperglycemia. The presence of FLUTD in a diabetic cat is not necessarily an indication that the cat will not go into diabetic remission. One recent paper showed that when an appropriate ultralow carbohydrate canned diet is used in conjunction with long-acting insulin, most newly diagnosed diabetic cats have a 70% to 90% chance of remission.156 The reader should refer to Chapters 24 and 32 for an in-depth discussion about treatment of DM and of FLUTD, respectively.
Tricyclic antidepressants have been found to be beneficial in the treatment of some humans with interstitial cystitis, and anecdotally, in a number of cats with FIC because of anticholinergic (including increasing bladder capacity), antiinflammatory (including preventing histamine release from mast cells), antiadrenergic, analgesic, and mood-altering effects.141 Potential side effects include somnolence, urinary retention, and increased liver enzymes. Liver function should be assessed prior to starting therapy, reassessed 1 month later, and then every 6 to 12 months while the cat is on treatment. It may be difficult to determine if liver enzyme elevations are caused by the diabetes or the tricyclic antidepressant; therefore control of the DM with gradual withdrawal of the agent may be required to determine whether the liver enzyme elevation is drug induced or not.
Heart Failure and Chronic Kidney Disease
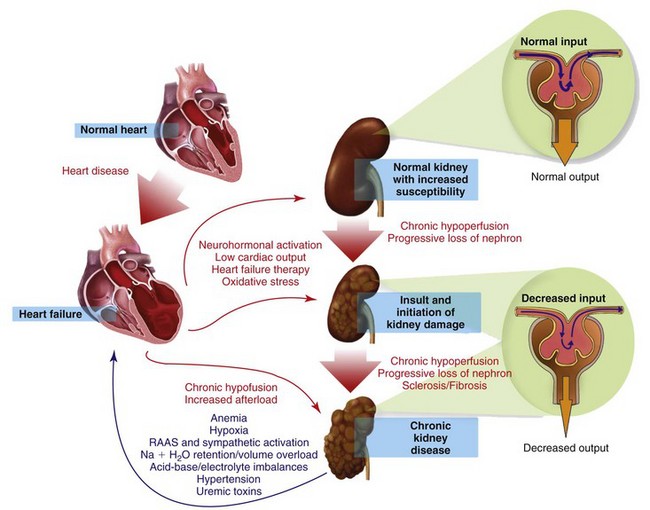
Myocardial disease and chronic kidney disease (CKD) are common disorders of the geriatric cat. The coexistence of heart failure (HF) and CKD is often associated with an adverse outcome, since combined cardiac and renal dysfunctions amplify progression of failure of the individual organ through a complex combination of neurohormonal feedback mechanisms.197
In humans, the term cardiorenal syndrome (CRS) has generally been reserved for declining renal function in the setting of advanced congestive heart failure, but recently, a more specific classification of CRS has been published.198 In this classification, CRS is divided into five subtypes that reflect the pathophysiology, time frame, and nature of concomitant cardiac and renal dysfunction (Box 35-1). The true existence of the negative spiral of primary CKD causing cardiac dysfunction is still unknown in small animals. Except for the consequences of hypertension, CRS subtypes 3 and 4 are probably uncommon in cats. For the purpose of this chapter, CRS will refer to subtype 2, assuming that relatively normal kidneys become dysfunctional because of concomitant HF. A special focus on the therapeutic strategies of concomitant CKD and HF will be presented.
Prevalence
Co-morbid dysfunction of the heart and kidney has been reported to be as high as 30% to 50% in hospitalized humans.100,161,196,209 A lower prevalence of 15% to 30% has been found in dogs with mitral valve disease.170,187 The true incidence of CRS in cats is unknown but also appears common. A study performed on 102 cats with hypertrophic cardiomyopathy reported 59% prevalence for azotemia as compared with 25% in an age-matched control population.80
Pathophysiology
The pathophysiology of CRS is complex and not completely understood. CRS occurs when worsening renal function limits diuresis despite a clinical volume overload associated with HF. The etiology of CRS is multifactorial and involves bidirectional interactions, effects, and reactions between the heart and kidneys. It includes activation of the renin angiotensin aldosterone (RAAS) and sympathetic systems, potentiation of oxidative stress, endothelial dysfunction, and defective nitric oxide metabolism (Figure 35-2). In chronic HF, long-term reduced renal perfusion is responsible for worsening renal function. However, hypoperfusion alone cannot explain progressive renal dysfunction as the cause of CKD. Numerous neurohormonal mechanisms are implicated and include vasoconstrictive (epinephrine, angiotensin II, endothelin), vasodilatory (BNP, nitric oxide), and inflammatory (C-reactive protein) mediators.4 Other contributing factors include the deleterious effects of uremia and acidemia on cardiac inotropy, the hypotensive effects of diuresis-associated hypovolemia, and RAAS blockade.164,171 Whether these mechanisms are responsible for CRS in cats remains speculative.
Diagnosis
The diagnosis of CKD is difficult in cats already being treated for HF. The diagnostic hallmark of isosthenuria in the presence of azotemia cannot be used in patients receiving diuretics, since they result in a lower USG. Also, mild or moderate prerenal blood urea nitrogen (BUN) elevation is expected in cats receiving diuretics, and a mild creatinine increase is also possible.17,170,187 However, a progressive rise of BUN, and especially creatinine with or without a decreasing USG, in a cat treated for chronic HF should alert the practitioner to potential development of CRS. Longitudinal assessment of serum creatinine is therefore important, and a progressive rise can indicate declining renal function even when values remain in the normal range. Other indices of CKD are hyperphosphatemia, hypokalemia, nonregenerative anemia, and proteinuria. The classical clinical signs of feline CKD (e.g., increasing polyuria/polydipsia [PU/PD], inappetence/anorexia, vomiting, and weight loss; see Chapter 32) should also be considered suspicious for CRS.
A promising tool in the diagnosis of CRS in cats is the measurement of serum amino-terminal probrain natriuretic peptide (Nt-proBNP). Indeed, this biomarker has been shown to offer powerful diagnostic and prognostic information in humans suffering from CRS.3,235 A commercial Nt-proBNP assay is now available in cats and could eventually prove useful in the diagnosis and management of CRS in this species.
Therapy
Treatment of CRS in cats is largely empiric, because no clinical trials have been completed. Box 35-2 summarizes the general approach to the cat presented with CRS. The goal is to recognize the syndrome, reverse it as much as possible, and deal with the renal consequences of chronic HF.