When the separation fails to occur, whether in part or totally, the condition is known as dysecdysis.
Dysecdysis
Dysecdysis is when ecdysis fails. It occurs in snakes, and to a lesser extent, lizards.
The causes of dysecdysis are many and varied, but can include any condition which can cause dehydration so reducing lymphatic fluid available to separate the skin layers. If the reptile is malnourished, dysecdysis may occur due to a lack of proteolytic enzymes. Alternatively old scars, a lack of an abrasive surface in the vivarium upon which to start the process or severe ectoparasitism may also cause dysecdysis.
Scale rot
Scale rot is a colloquial term for a range of conditions affecting the reptile skin which result in severe infections.
Septicaemic cutaneous ulcerative disease
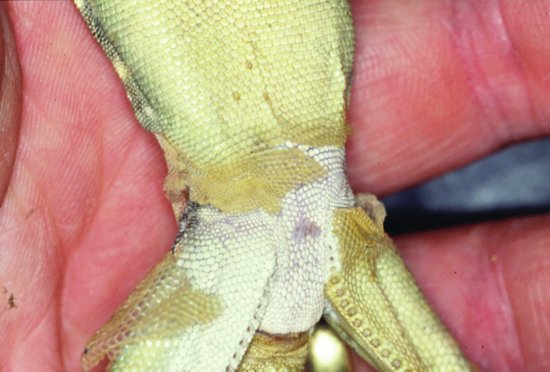
Septicaemic cutaneous ulcerative disease (SCUD) is seen in aquatic chelonians. Citrobacter freundii has been implicated which, once it has gained access to the bloodstream, produces ulcers in the skin and loosening of scales with debilitation and, in many cases, death. Other bacteria such as Pseudomonas and Aeromonas are also involved (Figure 21.3).
Blister disease
This is a form of scale rot seen in semi-aquatic species such as garter or water snakes. It can, however, occur in any species which is exposed to a persistently damp substrate. The outer layer of skin develops blisters of a clear fluid which become secondarily infected with environmental bacteria such as Aeromonas spp., Serratia spp., or Pseudomonas spp. These can progress to septicaemia. Occasionally these conditions may involve a fungus such as Aspergillus spp.
Abscesses
Reptile abscesses have recently been quoted as being more accurately termed fibriscesses (Huchzermeyer & Cooper, 2001). Instead of liquid pus, reptiles and birds form solid, caseous, dried pus surrounded by a thick shell of fibrous tissue. This is due to the lack of lysozymes and the secretion of huge amounts of fibrin into the abscess (Figure 21.4). These may form anywhere, but a common site in chelonians is the middle ear, causing a bulging of the ear drum (Figure 21.5). The pathogens involved are frequently Gram-negative species, although the presence of fungi has been well recorded.
Erythema, petechiae and ecchymoses
These three conditions may all be present at the same time or may appear individually.
Erythema is the reddening of skin tissues due to vascular congestion beneath. It can be seen in reptiles as a pink hue to the areas between scutes in the case of chelonians (Figure 21.3) and crocodilians, or the more generalised reddening of the skin seen in snakes. Erythema is often suggestive of septicaemia.
Petechiae are pin-point subepithelial haemorrhages. They can be seen in the mouth, where they are often associated with oral infection such as ‘mouth rot’. They may be seen over the body suggesting septicaemia. Other conditions producing petechiae include clotting deficiencies, for example, consumption of rodent prey which has itself consumed a warfarin-type poison.
Ecchymoses are larger areas of haemorrhage, and may be caused by severe local infection disseminated intravascular coagulation (DIC) or conditions mentioned above.
Overgrown beaks and claws
Overgrown beaks are common in chelonians, and are often due to a lack of abrasive foodstuffs or abrasive surfaces from which to eat them.
However, it should be noted that some species have perfectly normal apparently ‘long’ claws. An example is the male red-eared terrapin, which uses its claws as a display aid to attract and mate the female.
Pigment changes
An example of a physiologically normal colour change is seen in the Chamaeleonidae which will often darken in colour as they become stressed. Green iguanas often become yellow brown in colour as they become stressed or unwell, bearded dragons often become dark coloured and many of these species will exhibit darkening at the site of an injection. Areas of previous trauma may become whitened.
Ectoparasitic
Mites
The snake mite, Ophionyssus natricis appears as red to dark-coloured pin-head mites hiding under the overlapping edges of scales. They may be seen in water dishes in which the reptile has been bathing. They can cause severe irritation, pruritus and self-trauma, as well as causing anaemia and dysecdysis. In addition, they can transmit Aeromonas spp. bacteria, which may cause septicaemia and viruses, for example, inclusion body disease (IBD). Other mites include the cloacal mites of aquatic turtles of the family Cloacarus spp. In addition, there is of course the non-parasitic harvest mite or Neotrombicula autumnalis, which may be brought in on straw and hay bedding material. The adult itself is not an irritant but the six–legged larval stage is and may cause the reptile to traumatise itself.
Ticks
In the United Kingdom, Ixodes ricinus (the sheep or deer tick) and Ixodes hexagonous (the hedgehog tick) are seen. In tortoises, these are naturally the areas around the neck inlet and in front of the hind legs. They may cause local damage and can transmit pathogens. These include bacteria (Staphylococcus aureus, the cause of tick pyaemia and Borrelia burgdorferi, the cause of Lyme’s disease) as well as haemoparasites and viruses. Some of the soft-bodied ticks (Amblyomma marmoreum and A. sparsum) have been implicated in the transmission of Cowdria ruminantium the cause of heart water disease in mammals. These ticks are common on wild-caught imported reptiles.
Blowfly myiasis
Myiasis is a problem for any reptile kept in insanitary conditions or those with diarrhoea. Members of the blue bottle, black bottle and green bottle families are all capable of laying eggs on a tortoise. In peak conditions these can hatch into larvae or maggots within 1–2 hours. These then burrow away from the light, into the body of the tortoise causing severe trauma, infections, shock and ultimately death.
Leeches
Leeches tend not to be such a problem in the United Kingdom, but can affect aquatic species all over the world. The family Annelidae are the most dangerous, and cause large wounds which continue to bleed after the leech has detached. These may then become secondarily infected. They can also transmit haemoparasites, bacteria and viruses.
Traumatic and spontaneous damage
Traumatic damage can be due to attacks by predators or other reptiles, for example, males fighting over a female. In some species, for example, green iguanas, the male mounting the female during mating bites the shoulder area vigorously, causing open wounds. Dog attacks are common in tortoises (Figure 21.6). In the United Kingdom, it is unlawful to feed live vertebrate prey to any animal; but invertebrate live prey can be offered and these can still cause trauma to a sick or anorectic reptile. Unprotected heat sources frequently cause severe burns.
In some chelonians, scutes may be seen to lift off, often weeping clear fluid beneath them. This is frequently associated with underlying renal disease.
Iatrogenic causes of skin damage are also seen in some reptiles such as chelonians. This can occur after over-administration of vitamin A, which can cause sloughing of the epidermal layer on the head, neck and limbs, exposing the underlying dermis.
Tumours
Fibrosarcomas and squamous cell carcinomas of the head are seen in lizards and snakes. Melanomas are also seen in pigmented species such as the bearded dragon (Pogona vitticeps). Viral-induced fibropapillomas are frequently seen in green turtles (Chelonia mydas), where herpesviruses have been implicated. Another herpesvirus is a common cause of ‘grey-patch disease’ in this species, where circumscribed papular grey lesions appear over the whole body. In addition, papillomata have been regularly reported in Lacertidae (sand, wall and emerald lizards), occurring over the dorsum of the individual lizard.
Bacteria associated with skin problems
Gram-negative bacteria can cause skin abscesses, septicaemia and areas of skin sloughing. Others such as Mycobacteria spp. may be seen associated with the appearance of classical tuberculous lesions. The mycobacteria found are more commonly environmental ones, for example, Mycobacterium marinum (Girling & Fraser, 2007) and Mycobacterium chelonae, rather than the human or cattle TB organisms.
Dermatophilus congolensis has been associated with skin disease in a number of lizards and snakes and D. cheloniae has been isolated from captive chelonians (Masters et al., 1995).
Dermabacter spp. related bacteria have been associated with a proliferative cheilitis syndrome in Agamid lizards (Koplos et al., 2000). This has also been seen to cause proliferative lesions around the cloaca and over the legs (Pasmans et al., 2010) and is now thought to be due to the bacterium Devriesea agamarum. Gram-negative bacterial infections of the hind feet of Savannah monitor lizards seems to be another common condition and is associated with an overly abrasive and damp substrate leading to foot abrasion and secondary infection (Stahl, 2003).
Fungal
The incidence of fungal infections in reptiles has been quoted between 0.4% (Austwick & Keymer, 1981) and 4% (Jacobson, 1980). These include: Aspergillus spp. (Austwick & Keymer, 1981; Tappe et al., 1984; Girling, 2002; Girling & Fraser, 2009); Mucor spp. (Lappin & Dunstan, 1992; Heatley et al., 2001); Paecilomyces spp. (Heard et al., 1986; Schumacher, 2003; Diaz-Figueroa et al., 2008); Candida albicans (Austwick & Keymer, 1981; Migaki et al., 1984); Fusarium spp. and Chrysosporium spp. (Austwick & Keymer 1981; Schumacher 2003); Sporothrix schenckii, Pestalotia pezizoides and Geotrichum candidum (Cheatwood et al., 2003); Beauveria bassiana (Gonzalez Cabo et al., 1995); Penicillium griseofulvum (Oros et al., 1996); Alternaria spp. (Rossi & Rossi, 2000); Cryptococcus spp. (Hough, 1998); Scolecobasidium humicola (Weitzman et al., 1985) and the primary fungal pathogen the Chrysosporium anamorph of Nannizziopsis vriesii (CANV) in bearded dragons, brown tree snakes and chameleons (Pare et al., 1997; Nichols et al., 1999; Bowman et al., 2007; Abarca et al., 2009). CANV produces the so-called ‘yellow skin disease’ of bearded dragons and may be fatal to a wide range of reptiles (Figure 21.7).
Many of these fungal infections appear to enter the reptilian body via the skin (Austwick & Keymer, 1981). With increasing use of antibiotic therapy in pet reptiles and the discovery of new fungal species associated with disease (e.g., CANV), the incidence of fungal disease appears to be on the increase (Fraser & Girling, 2004).
Viral
There are more and more cases of viral associated disease being diagnosed in reptiles each day. Herpesviruses causing grey patch disease have been found in turtles, papilloma viruses in lizards, and pox viruses in caimans (a member of the family Crocodylia).
Hereditary
Many species exhibit scale and skin abnormalities. These vary from cleft palates, to failure of scales to develop at all. In addition, many colour variations have been specifically bred for.
Hyperthyroidism
This has been diagnosed in green iguanas and corn snakes, although it is rare. It is associated with an increased turnover of skin and so an increase in ecdysis. It may also be associated with loss of dorsal spines in iguanas, tachycardia, increased aggression, polyphagia and weight loss overall (Hernandez-Divers et al., 2001). Normal thyroid reference ranges are difficult to find, but 0.21–6.78 nmol/L have been reported for Calotes and Sceloporus lizards.
Hypothyroidism
This has been reported in giant chelonia (Frye, 1991). It may also be due to the presence of goitrogens in the diet such as brassicas. Myxoedema and heart failure are the most commonly seen clinical signs.
Thermal burns
Reptiles can sense pain but do not appear to respond to thermal pain in the way that birds and mammals do; that is, they do not remove themselves from the heat source even when it is resulting in severe burns. Unprotected heat lamps which allow reptiles close contact are dangerous, as are so-called ‘hot-rocks’ when the thermostat breaks. Full skin thickness burns and even death of the reptile are not uncommon (Figure 21.8).
Figure 21.8 Full skin thickness burns can be seen where heat lamps are left unprotected as in this monitor lizard.

Hypovitaminosis A
This is mainly seen in aquatic chelonians and chameleons. The presenting signs include ocular oedema, xerophthalmia, middle ear abscessation, hyperkeratosis, lethargy and anorexia. It is preferable that vitamin A supplements should be given orally to minimise toxicity. Values of 200 IU/30 g bodyweight have been quoted dosed twice, 7 days apart (Harkewicz, 2001).
Hypervitaminosis A
This can occur if parenteral vitamin A is given in doses exceeding 10,000 IU/kg. Initially it presents as dry skin, but may progress to whole skin sloughing. Management is as for thermal burns.
Hypovitaminosis C
This has been reported by Frye (1991) as resulting in spontaneous splitting of the skin in anorexic/cachexic pythons and may be associated with a collagen defect. Injections of 10–20 mg/kg vitamin C per day are advised.
Hypovitaminosis E
This has been reported in caimans and other reptiles which eat marine fish. The presence of free radicals in oily frozen marine fish can result in a relative vitamin E deficiency which can lead to steatites, skin sloughing, and necrosis of fat and secondary infections. Dietary supplementation with vitamin E at 1 IU/kg until resolution of signs is recommended.
Biotin deficiency
This may be seen in reptiles which consume large amounts of eggs, such as some snakes and monitor lizards. Unfertilised eggs contain avidin, an anti-vitamin to biotin which may lead to a relative deficiency. The main signs are neurological, but fracturing and splitting of keratin structures such as scales and claws and beaks along with abnormal sloughing may be seen. Removal of eggs from the diet results in a cure in most cases.
Hypocalcaemia/hypovitaminosis D
This can result in shell deformities in chelonians. See the section on musculoskeletal diseases for further information. In addition, calcinosis cutis and circumscripta have been reported in reptiles with multifocal crusting and thickened skin which can then ulcerate. These two conditions are often related to calcium imbalances – more often hypercalcaemia, but also to areas of skin damage.
Digestive disease
Oral (mouth rot)
‘Mouth rot’ is the colloquial term for stomatitis, commonly seen in many reptiles particularly in snakes and chelonians.
The causes are many and varied. It can be caused by secondary infections with Gram-negative bacteria. The initial cause of the damage can be rubbing of the snout on vivarium glass, particularly in snakes. The use of opaque tape stuck to the outside of the glass helps the snake to ‘see it’, preventing this injury.
Stomatitis may also be due to the overzealous force-feeding of anorectic reptiles, to other disease or stresses leading to reduced immune system function. Uric acid crystals deposited around the base of the teeth, due to visceral gout and renal failure can allow infections to occur. Herpesvirus and iridovirus infections in terrestrial tortoises are also a cause with oral ulceration and diphtherisis. Lytic agent X – a suspected virus – has also been associated with upper digestive tract lesions in chelonia. Devriesea agamarum, a bacterium, has been reported in agamid lizards as a cause of proliferative cheilitis, pericloacal disease and dermatitis.
Diagnosis is made on impression smears and bacterial swabs of samples. A polymerase chain reaction (PCR) for diagnosing chelonian herpesvirus has been produced at the RVC London, and reptile cell lines are available through the AHVLA Weybridge to attempt virus isolation.
Alternatively, electron microscopy of samples may aid the diagnosis.
Herpesvirus may also affect the central nervous system and organs such as the liver, kidneys and gonads. Depression and neurological signs are possible.
Periodontal disease
This is particularly common in Agamidae and Chameleonidae which possess acrodont teeth. These teeth are not replaced when lost during the course of the lizard’s life. This is as opposed to pleurodont teeth (e.g., the Iguanidae) where the teeth are continually replaced. If inappropriate food is provided, such as soft fruit, which adheres readily to the teeth and rots, periodontal disease will develop. As the species with acrodont teeth cannot shed and replace the teeth, infection, often with osteomyelitis ensues.
Vomiting and regurgitation
In snakes, regurgitation may simply occur due to rough handling, particularly soon after the snake has eaten. Snakes will also regurgitate if fed, or force-fed and then kept at sub-optimal environmental temperatures. Lizards and chelonia rarely vomit.
Parasitic causes of vomiting and regurgitation
In snakes, one of the most important stomach diseases is cryptosporidiosis due to a single-celled parasite, Cryptosporidia serpentes, which invades the outer membrane of cells lining the stomach. With time, it causes a thickening of the stomach, narrowing the lumen and so reducing its elasticity and digestive powers and causing vomiting. In lizards, cryptosporidiosis mainly affects the intestines, making vomiting less likely. Cryptosporidiosis was implicated in an outbreak of vomiting in juvenile Hermann’s tortoises (McArthur et al., 2004). It is therefore currently thought that there is a lizard-, snake- and chelonian-specific form of Cryptosporidia spp.
Diagnosis is made on clinical signs and demonstrating oocysts shed in the faeces or recovered from a stomach wash, or biopsy of the stomach wall. In snakes, the course of the disease can vary from 4 days in severe cases to 2 years in chronic ones. Externally, the snake often shows signs of a mid-body swelling, due to the thickening of the stomach wall. The parasite is passed directly from one snake’s faeces or regurgitated fluids to another. The condition is difficult to treat and frequently results in the death of the snake (Figure 21.9).
Other forms of parasitism may cause vomiting or regurgitation in snakes. For example, Kalicephalus spp. (the snake hookworm), which may cause extensive ulceration of the digestive system; ascarids; and in the python family, the tapeworm Bothridium spp., have been reported.
Bacterial and fungal causes of vomiting and regurgitation
Bacterial and fungal infections, granulomas and abscesses may also cause damage to the stomach, or pressure on it, and so lead to regurgitation or vomiting.
Viral causes of vomiting and regurgitation
Inclusion body disease, a retrovirus, has been associated with chronic regurgitation in boids although there are usually other clinical signs such as respiratory disease and neurological disease.
Tumours
Stomach tumours, for example, gastric adenomas and adenocarcinomas, have been reported and may cause swelling.
Intestinal disease
Parasitic causes of intestinal disease
Snakes
Species involved include the hookworm Kalicephalus spp.; ascarids; strongyles such as Strongyloides spp.; and tapeworms such as Bothridium spp. in pythons; and Ophiotaenia spp. in garter and water snakes.
Entamoebiasis caused by the single-celled parasite Entamoeba invadens can cause diarrhoea in snakes. Its life cycle is direct; that is, spread directly from one reptile to another. After incubating in the lining of the small intestine, each infective cyst produces eight uninucleate amoebae which invade cells lining the large intestine of the same snake. The amoebae may also penetrate into the blood stream and so end up in the liver, causing necrotising hepatitis. Diagnosis is by finding the small amoebae or cysts in the faeces (× 400 magnification is required).
Other single-celled parasites of snakes include the flagellate family, (e.g., Trichomonas spp.). Symptoms again include large bowel distension with fluid and gas, diarrhoea and increased thirst.
Chelonians
The ascarid family, for example, Angusticaecum spp. (10–20 cm in length!) is most significant. Oxyurid pinworms may be asymptomatic, but some may be large enough to debilitate a tortoise, particularly prior to and subsequent to hibernation. Roundworms are passed directly from chelonian to chelonian in the faeces in the infectious egg form, and may be diagnosed by the detection of these eggs in a faecal smear.
The most important group of intestinal parasites of Chelonia is the flagellate family. These include Trichomonas and Hexamita spp. The symptoms produced are rapid in onset and include anorexia, occasionally diarrhoea and frequently, the passage of undigested food in the faeces. There is often polydipsia, due partly to the inability of the damaged intestines to absorb water, and partly, in the case of Hexamita parva, due to kidney damage, as this organism migrates up the ureters from the cloaca into the kidneys. Diagnosis is made by viewing the very fast-moving organisms microscopically using × 400 magnification, although a fresh faecal specimen is required. Transmission is faeco-oral. The prognosis may be extremely poor for any chelonian severely parasitised by this organism, due to its ability to cause irreparable damage to the intestinal mucosa.
Another family of motile protozoa afflicting Chelonia are ciliates such as Balantidium coli and Paramecium spp. They may be seen normally in the stool of healthy animals, but they may be present in such large numbers as to cause weight loss and intestinal damage.
Entamoeba invadens has caused large intestinal and hepatic disease in herbivorous chelonians. However, this condition is less common than in snakes.
As mentioned, Cryptosporidia spp. can affect the small intestine and stomach of chelonia, and has been implicated in pancreatitis as well.
Lizards
Oxyurid nematodes are common. Due to the direct life cycle of these parasites, levels may become very high enough to produce intestinal damage and debilitation. Diagnosis is by finding the eggs in the faeces (Figure 21.10).
Figure 21.10 Oxyurid nematode infections are common in reptiles and may be diagnosed by finding these classical eggs in the faeces.
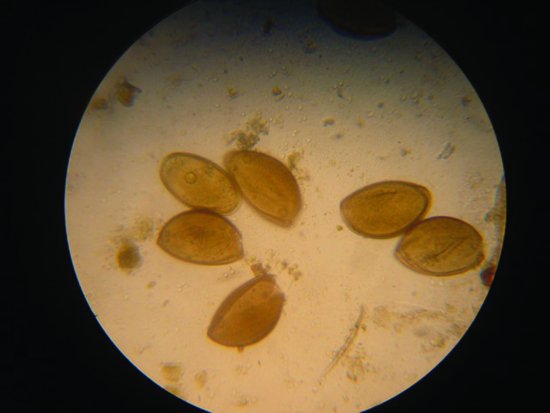
The coccidian family are also important parasites in lizards, particularly geckos and chameleons. Clinical signs include anorexia, weight loss, diarrhoea, dysentery and general debilitation. Isospora amphiboluri is a cause of stunting and runting in bearded dragon neonates. Diagnosis is by finding the typically isosporean oocysts in the faeces, and disease spread is direct.
Intestinal cryptosporidiosis is a problem in geckos. Symptoms include weight loss, diarrhoea and anorexia and typically affect young hatchlings that basically fail to thrive. The pathology has been described above.
Entamoeba invadens may also be seen in carnivorous lizards, producing colic and progressive weight loss with diarrhoea.
Bacterial causes of intestinal disease
In all species, but particularly snakes and chelonians, members of the zoonotic family Salmonellae are commonly recovered. Current advice is that providing they are not causing clinical disease, no antibiotics should be given. This is to prevent antibiotic resistance, and on a practical basis no one has satisfactorily proven that such treatment can clear a reptile permanently of the bacterium.
Salmonella spp. may be carried by perfectly healthy individuals, but during other disease processes, such as parasitism, or during periods of stress, they can become opportunistic pathogens. Other bacteria found in the normal digestive system of reptiles can act as opportunistic pathogens, including many members of the E. coli family, Pseudomonas spp., Campylobacter spp., Clostridia spp., and Aeromonas spp.
Fungal causes of intestinal disease
Published reports of gastrointestinal fungal disease are rare, but fungal overgrowth appears to be a relatively common finding. Documented cases of intestinal fungal overgrowth include Penicillium, Basidiobolus ranarum and Paecilomyces.
Physical causes of intestinal disease
Foreign bodies are not uncommon in chelonians and lizards, which may consume stones, sand, soil or any other substrate present. Surgery may be required to remove some foreign bodies, although many may be passed with the aid of oral liquid paraffin and fluid therapy.
Other physical obstructions include tumours within the intestines. These are not uncommon, particularly in snakes, and may present as a swelling, or produce symptoms such as vomiting, diarrhoea, constipation or anorexia.
Lead poisoning
This has been reported in chelonian such as common snapping turtles (Chelydra serpentina) and spur-thighed tortoises (Testudo graeca). Clinical signs included ileus, anaemia, lethargy and renal failure. Radiography will highlight larger lead particles.
Liver disease
Snakes
Possible causes of liver damage in snakes (Entamoeba invadens) have already been mentioned above.
Other parasitic causes include some forms of the coccidian family which gain access to the liver from the small intestine via the bile ducts.
A herpesvirus and an adenovirus have been isolated from damaged snake livers.
Chelonia
The protozoan Hexamita parva has been associated with intestinal, renal and hepatic disease in Chelonia.
Hepatic lipidosis is common in tortoises fed inappropriate high-fat diets. Fat becomes deposited within the liver cells themselves, enlarging the liver and, more importantly, severely affecting its function. This can lead to jaundice, anorexia and death.
Post-hibernation jaundice is often a temporary finding in tortoises however it may persist, indicating liver damage due to any one of the above or due to hepatitis from bacteria such as Salmonella spp. and Aeromonas hydrophila. In addition some forms of herpesviruses and iridoviruses have been isolated from damaged tortoise and other chelonian livers.
The liver may become calcified due to over-supplementation of the diet with calcium and vitamin D3.
Lizards
Bacterial hepatitis due to Salmonella spp., Aeromonas spp. or Pseudomonas spp. has all been recorded. These may or may not be associated with heavy worm burdens, many of which may migrate through or to the liver, often carrying bacteria with them.
Viral hepatitis has been reported due to adenoviruses in bearded dragons (Pogona spp.). It is generally seen in young animals and often associated with coccidiosis resulting in poor thriving young. Serological tests are available for detection of this disease as is a PCR test. Herpesviruses have been associated with hepatitis as well as pneumonia in iguanids and agamids. Yellow-green urates may be seen in both snakes and lizards associated with hepatic dysfunction due to the presence of increased levels of biliverdin.
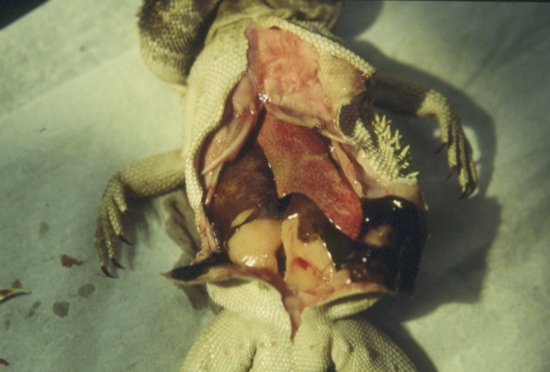
Hepatic lipidosis, or fatty liver syndrome, has been well documented in lizards, particularly monitors and bearded dragons. This is frequently seen when fed inappropriate high-fat diets. In bearded dragons, the feeding of excessive amounts of insects to adults (which are supposed to become more herbivorous once they reach maturity) has also been implicated (Figure 21.11). In lizards with pre-ovulatory stasis, lipidosis occurs due to the high levels of circulating yolk lipids. Lipidosis can lead to jaundice, anorexia and death. The liver may become calcified due to over-supplementation of the diet with calcium and vitamin D3.
Figure 21.11 Hepatic lipidosis is common in species such as the bearded dragon (Pogona spp.). Note the tan coloured liver and fat deposits.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree