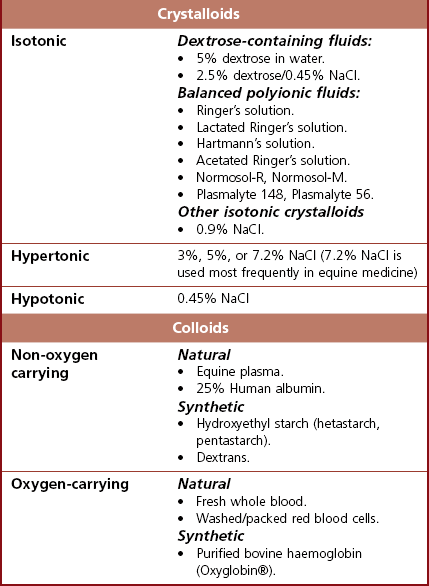
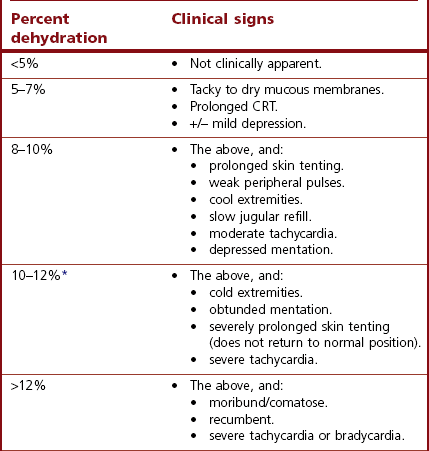
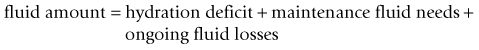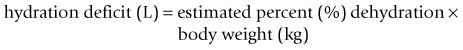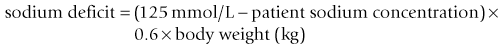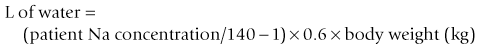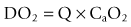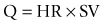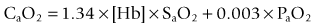Chapter 26 26.1 Principles of fluid therapy 26.2 Pathophysiology and management of shock 26.3 Administration of blood products in equine critical care 26.4 Nutritional support in the critically ill horse and foal 26.5 Evaluation and management of the sick neonatal foal 26.6 Management of acute respiratory distress 26.7 Cardiopulmonary cerebral resuscitation (CPCR) • Fluid therapy is most commonly used to restore and/or maintain hydration and blood volume. • Fluid losses and dehydration may occur as a direct result of the primary disease, such as gastrointestinal or urinary losses in colitis or renal failure or loss in acute or chronic haemorrhage, or may be due to decreased water intake in patients with anorexia or dysphagia. • Enteral intake of feed and water is also often restricted for periods of time in the management of horses with certain types of colic, in which case intravenous fluids must be administered to prevent dehydration. • Occasionally, specific types of fluids are administered to euhydrated patients to correct electrolyte or acid-base derangements, to provide colloidal support to hypoproteinaemic patients, or to provide oxygen carrying capacity in anaemic patients. • Constructing an appropriate fluid plan for a critically ill patient is both a science and an art. • When forming a fluid plan, four key components must be addressed: 1. fluid volume to be administered. 2. type of fluids to be administered. 3. route of fluid administration. • In addition, the patient’s status and response to the initial plan must be monitored closely – sometimes hourly or even more frequently in critically ill horses – and the fluid plan revised as the patient’s condition changes. In general, the amount of fluids to be administered is determined by the following formula: The % dehydration typically ranges from 5 to 12% and is estimated based on physical examination and clinicopathological findings. Typical clinical findings in horses with varying degrees of dehydration are shown in Table 26.1. Table 26.1 Clinical signs associated with dehydration in the horse *Note: ≥10–12% dehydration is fatal if not addressed rapidly and aggressively. • Fluids with a similar electrolyte composition to plasma are described as ‘balanced’. • Crystalloids are solutions that contain both electrolyte and non-electrolyte solutes that can move freely between the extracellular and intracellular fluid compartments. Crystalloids may be isotonic, hypertonic, or hypotonic relative to plasma, and may be balanced or unbalanced. Examples of crystalloid fluids used commonly in equine medicine are shown in Table 26.2. Isotonic crystalloid fluids are administered primarily to restore and maintain hydration and correct electrolyte derangements. Hypertonic crystalloids are used to rapidly increase intravascular volume in patients with shock, but must be followed by an appropriate volume of isotonic fluids (see section on ‘Pathophysiology and management of shock’). Hypotonic fluids are used infrequently; they are used in patients with increased plasma osmolality (e.g. hypernatraemia). • Colloids are solutions that contain high-molecular-weight solutes that cannot leave the vascular space, and thus serve to increase colloidal oncotic pressure. The most common colloids used in equine medicine are equine plasma or synthetic colloids such as dextrans and hydroxyethyl starch (hetastarch). Colloids are most often used in patients with shock or severe hypoproteinaemia (see section on ‘Pathophysiology and management of shock’). • Oxygen-carrying fluids include whole blood, packed red blood cells, and purified bovine haemoglobin products (e.g. Oxyglobin®). Oxygen-carrying fluids are administered to patients with decreased oxygen-carrying capacity secondary to severe acute haemorrhage or anaemia. These products contain large macromolecules and are thus colloids, but as they do less to increase colloidal oncotic pressure, compared to plasma and synthetic colloids, they are not typically used in this manner. Administration of these products is covered in more detail below in the section on ‘Administration of blood products in equine critical care’. • Intravenous fluid administration. Figure 26.1 Jugular (a) and lateral thoracic (b) intravenous catheters sutured in place in adult horses. • Enteral fluid administration. • Other routes of administration. • Shock-rate intravenous fluid administration (see section on ‘Pathophysiology and management of shock’ for more detail) • Maintenance intravenous fluid administration. Hyponatraemia: CNS signs (altered mentation, recumbency, seizures) are typically seen with serum sodium concentrations <120 mmol/L, but sodium concentrations may be much lower if hyponatraemia develops slowly. The sodium deficit is calculated as follows: • If neurological signs are present, aim to correct the sodium deficit over approximately 6 hours. • If the horse is asymptomatic or if hyponatraemia is known to have been present for a prolonged period of time (several days or more), the deficit should be corrected over 24–48 hours (no faster than 0.5 mmol/hour). • An appropriate fluid type, typically 0.45% or 0.9% NaCl or a balanced polyionic isotonic crystalloid, is selected based on the sodium content of the fluid and the rate at which fluids are being administered, to avoid replacing sodium too quickly. • It is vital to monitor serum sodium concentrations closely (as often as every few hours initially) during therapy to ensure that the correction is not too rapid. • CNS signs are frequently observed with serum sodium concentrations >160 mmol/L. • Hypernatraemia is corrected by administration of fluids containing free water. • The amount of these fluids to administer is calculated by the following formula: • It is vital that hypernatraemia be corrected slowly, no faster than 0.5 mmol/hour. If the patient is dehydrated and requires fluid administration at a faster rate, fluids containing sodium should be used to avoid too rapid correction. Hypokalaemia: Since potassium is primarily an intracellular cation, serum potassium concentration is a relatively insensitive indicator of total body potassium status. However, severe hypokalaemia can result in muscle weakness and cardiac arrhythmias; thus, hypokalaemia should be corrected if serum potassium concentration is <3 mEq/L, and potassium should be supplemented even in normokalaemic patients with prolonged anorexia or if fluids containing dextrose or bicarbonate are administered, as these can lead to rapid intracellular movement of potassium ions. Hyperkalaemia: Hyperkalemia can result in life-threatening cardiac arrhythmias, and thus requires rapid and aggressive treatment. • Intravenous administration of sodium bicarbonate (1 mEq/kg diluted in 5% dextrose), 5% dextrose in water (0.5 mL/kg), or regular insulin (0.1 IU/kg) and 5% dextrose (0.5 mL/kg) may be used to drive potassium intracellularly and decrease serum potassium concentrations. • If serum potassium concentration is >6 mmol/L or electrocardiogram abnormalities (e.g. tall peaked T waves, bradycardia, widened QRS complexes, prolonged PR intervals, or atrial standstill) are present, calcium gluconate (4 mg/kg IV diluted in 1 L 0.9% NaCl) should also be administered intravenously. This has no effect on the serum potassium concentration but has cardioprotective effects while the above therapy is initiated. • Administration of non-potassium-containing intravenous fluids at 1–2× maintenance rates also helps to eliminate excess potassium via urinary excretion. Hypocalcaemia: Hypocalcaemia is frequently encountered in horses with GI disease, periparturient mares, and in blister beetle (cantharidin) toxicosis. • If severe (total calcium less than ~6.0 mg/dL, ionized calcium <1 mmol/L), hypocalcaemic tetany is likely to be observed, and may progress to recumbency and seizures in severe cases. • Specific calcium correction formulas are not established for horses. In a clinical setting, hypocalcaemia is typically corrected by intravenous administration of 1 mL/kg 23% calcium gluconate. Calcium should be diluted in isotonic fluids or administered very slowly intravenously to avoid development of cardiac arrhythmias. Hypomagnesaemia: Hypomagnesaemia often accompanies hypocalcaemia in horses and is frequently seen in anorectic horses or those with GI disease. • Clinical signs associated with hypomagnesaemia include tachycardia, cardiac arrhythmias, muscle weakness, and sedation. Interestingly, the clinical signs of hypermagnesaemia are essentially identical. • Estimation of total body magnesium status is difficult. In general, horses with total serum magnesium concentration less than about 1–1.5 mg/dL may show clinical signs and should receive magnesium supplementation. • Magnesium supplementation in severe hypomagnesaemia is usually provided with intravenous magnesium sulphate at 50–100 mg/kg (25–50g per 500 kg horse) every 12–24 hours. Magnesium sulphate should be diluted in at least 1 L of sterile fluids for intravenous use. • Mildly to moderately dehydrated horses often exhibit a mild metabolic acidosis and hyperlactataemia associated with poor tissue perfusion that resolves without specific treatment when dehydration is corrected. • However, in severely acidotic horses or foals, such as those with a blood pH < 7.1, serum bicarbonate concentration <15 mmol/L, or a base deficit >10–15 mEq/L, alkalinizing therapy with sodium bicarbonate is indicated. • The bicarbonate deficit in adult horses is calculated as follows: • Metabolic alkalosis is much less common in horses than metabolic acidosis, but may be seen in horses with excessive sweating or gastric outflow obstructions due to concurrent hypochloraemia. • The alkalosis typically resolves without specific therapy when the hypochloraemia is corrected with administration of isotonic balanced crystalloids, though administration of acidifying fluids such as 0.9% NaCl can be helpful in severe cases. • Several equations are important to understanding the factors that can result in inadequate oxygen reaching the cell. Understanding what is causing inadequate oxygen delivery allows targeted therapy to improve oxygen delivery during the treatment of shock. • Oxygen delivery (DO2) is the product of cardiac output (Q) and arterial oxygen content (CaO2): Hypovolaemic shock is caused by an insufficient circulating blood volume. • Blood volume can be lost as whole blood (haemorrhage) or free water (with or without electrolytes and/or proteins). • The fluid can be lost outside of the body or sequestered within the body, but outside of the circulation (third space). • Prolonged lack of fluid intake can also result in hypovolaemia. • Examples of conditions that can be associated with hypovolaemic shock include: • Circulating endotoxin interacts with a circulating host protein called LPS-binding protein (LBP) and a circulating or cell membrane receptor called CD14. This LPS–LBP-CD14 complex then binds and activates a cell surface receptor on leukocytes and endothelial cells called toll-like receptor 4 (TLR-4). • Activated TLR4 initiates an intracellular signalling cascade that activates the transcription factor NFκB. NFκB enters the nucleus and stimulates the transcription of many proinflammatory mediators such as TNF-α, IL-1 and IL-6. These inflammatory mediators stimulate leukocyte adhesion and activation and endothelial cell activation, cause increased production of PGE2, ACTH, acute phase proteins, eicosanoids, and NO, and induce the release and activation of arachidonic acid metabolites, platelet-activating factor, reactive oxygen species, histamine, kinins and complement. Many of these substances have effects on vasomotor tone and vascular permeability, which can result in altered blood flow and distributive shock. • Endotoxaemia can occur secondary to a variety of diseases in horses, including colitis, pleuropneumonia, and retained foetal membranes. Sepsis: Sepsis is a condition that occurs in bacteraemic patients (those with bacteria in the bloodstream) when the body’s inflammatory response to the infection becomes systemic (resulting in the systemic inflammatory response syndrome – SIRS). • The inflammatory mediators associated with sepsis/SIRS include many of the same cytokines and result in many of the same effects as are described above for endotoxaemia. • Sepsis is most common in foals (see Chapter 20), but may also occur in adult horses secondarily to bacterial infection or compromise in the gastrointestinal mucosal barrier. Anaphylaxis: This is a systemic hypersensitivity reaction that results in mast cell degranulation, and thus, the release of large amounts of histamine, prostaglandins, and leukotrienes. Severe vasodilation can occur as a result. Anaphylaxis is uncommon in horses but can occur with drug or blood product administration. • It is important to remember that these compensatory mechanisms are not without consequence (such as decreased perfusion to ‘less vital’ organs like the gastrointestinal tract), and will eventually become exhausted. • These compensatory mechanisms include multiple physiologic responses that are triggered when the body detects the decreases in oxygen delivery, circulating volume, and mean arterial pressure including: Stage II: Early decompensated shock: • Compensatory mechanisms are unable to meet energy needs of tissue resulting in increased anaerobic metabolism, lactic acidosis, and organ dysfunction. • Clinical signs: progressive tachycardia and tachypnoea, prolonged CRT, a ‘toxic line’ on mucous membranes, cold extremities, decreased urine production, and altered mentation. Stage III: Late decompensated (also known as irreversible) shock: • As anaerobic metabolism continues, sympathetic arterial and venous constriction is overwhelmed, resulting in blood pooling in venules, fluid leaking into interstitium, and activation of inflammatory cells. Eventually, this leads to organ failure and death. • Clinical signs: marked hypotension, bradycardia, circulatory collapse, pale/grey mucous membranes, and organ failure. • This stage is often fatal, even with aggressive treatment. As described above, cardiac output is the product of heart rate and stroke volume. The majority of therapies utilized in most types of shock are directed at restoring stroke volume rather than altering heart rate, unless a pathological cardiac arrhythmia is present (see Chapter 7). • Isotonic crystalloids, which are widely available and easy to use, can be administered at a shock dose of 90 mL/kg. • Hypertonic saline solution (2–4 mL/kg IV bolus) can also be used to rapidly increase intravascular volume (2–4 L for every 1 L of hypertonic saline solution administered). • Colloids (10–20 mL/kg) can also be used for fluid resuscitation for rapid volume expansion. Fluid administration is not without risk in certain conditions associated with shock. • Leakage of fluid into the interstitial space can occur with administration of crystalloids in diseases associated with hypoproteinaemia (colitis) or impaired vascular function (endotoxaemia, lung contusions, SIRS). • In cases of uncontrolled haemorrhagic shock, rapid volume expansion can disrupt tenuous blood clots. In these cases, low-volume fluid resuscitation coupled with the administration of blood/plasma products to support coagulation is preferable to rapid, high-volume fluid resuscitation. • In equine critical care, pressors and inotropes are most often used in neonatal foals at referral institutions, and are very rarely used in standing adult horses. • However, as many of the disease conditions associated with shock in horses are associated with decreased vasomotor tone and may have effects on the myocardium, it is possible that some cases could benefit from such pharmacological support. • Treatment with pressors and inotropes requires intensive monitoring and care, and detailed discussion of their use is beyond the scope of this text. In cases where the oxygen-carrying capacity is limited by anaemia, administration of red blood cells (whole blood or packed red blood cells) or purified haemoglobin dramatically improves the oxygen content of the blood (see section on ‘Administration of blood products in equine critical care’). Many of the clinical signs of shock are monitored best with serial physical examination. • Monitoring urination can also provide information on perfusion and hydration. • Assessment of plasma lactate concentration and blood gas parameters can also be helpful. • Central venous pressure (CVP) is the pressure within the greater vessels feeding the heart and is measured via a pressure transducer attached to a catheter placed through the jugular vein into the cranial vena cava or right atrium. • Arterial blood pressure can be measured directly with an arterial catheter or indirectly using oscillimetry or Doppler flow. Mean arterial pressure should be approximately 100 mmHg in a standing adult horse and above 60 mmHg in a foal. Plasma is used primarily in the following circumstances: • Provision of colloidal oncotic support to patients with hypoproteinaemia or shock. • Provision of coagulation factors in coagulopathic patients. • Provision of endogenous ‘anti-endotoxic’ factors such as anti-LPS antibodies and provision of immune support to patients at risk for, or with signs of, endotoxaemia or sepsis. • Provision of immunoglobulins to neonatal foals at risk for, or with evidence of, failure of passive transfer or immunity. • Fresh plasma, rather than frozen plasma or whole blood, is indicated when active functional platelets but not erythrocytes are required, such as in horses that are thrombocytopenic but not anaemic. • Concentrated platelet preparations (platelet-rich-plasma) are often used in humans and small animals, and can be prepared with equine plasma, but are rarely used in horses due to the associated expense. • Washed erythrocytes are most often administered to neonatal foals with neonatal isoerythrolysis (NI) (see Chapter 20). In these cases the dam’s blood is most often used. The dam’s plasma contains antibodies against the foal’s erythrocytes, so it must be removed by washing the cells prior to the transfusion. • Purified bovine haemoglobin preparations (such as Oxyglobin®) can be used in horses requiring oxygen-carrying capacity for which a compatible blood donor is not available. • Hyperimmune plasma or serum products consist of the plasma or pooled serum from horses that have been hyperimmunized against a particular substance to provide disease-specific antibodies in high concentrations.
Common problems and techniques in equine critical care
26.1 Principles of fluid therapy
Components of a fluid plan
Fluid volume
Type of fluid
Route of fluid administration
 This route is used most often in equine critical care, as it permits rapid administration of large volumes of fluids.
This route is used most often in equine critical care, as it permits rapid administration of large volumes of fluids.
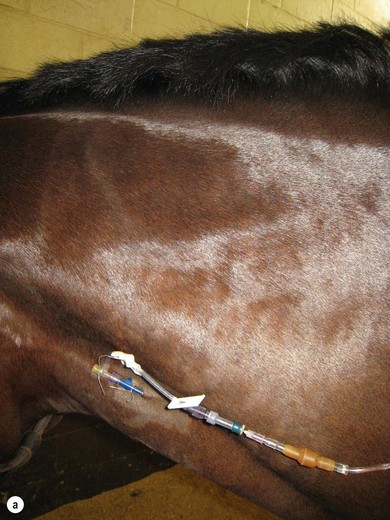
 In horses and foals, intravenous access is most often achieved through catheterization of one or both jugular veins. In adult horses, the lateral thoracic veins may also be used, though fluids cannot be administered quite as rapidly (Figure 26.1).
In horses and foals, intravenous access is most often achieved through catheterization of one or both jugular veins. In adult horses, the lateral thoracic veins may also be used, though fluids cannot be administered quite as rapidly (Figure 26.1).

 The cephalic veins are occasionally used if jugular or lateral thoracic vein access is limited, but cephalic catheters can be dangerous to place in some fractious horses and are typically difficult to maintain for long periods.
The cephalic veins are occasionally used if jugular or lateral thoracic vein access is limited, but cephalic catheters can be dangerous to place in some fractious horses and are typically difficult to maintain for long periods.
 This method is typically inexpensive and is appropriate for patients that can tolerate oral fluids (e.g. patients that are not refluxing and that do not have intestinal disease that would impair absorption of oral fluids) and for those that do not require rapid administration of large volumes of fluid (e.g. severely dehydrated patients or patients in shock).
This method is typically inexpensive and is appropriate for patients that can tolerate oral fluids (e.g. patients that are not refluxing and that do not have intestinal disease that would impair absorption of oral fluids) and for those that do not require rapid administration of large volumes of fluid (e.g. severely dehydrated patients or patients in shock).
 Enteral fluid therapy is typically administered to horses via a nasogastric tube; an indwelling nasogastric tube may be maintained for repeat administration or continuous infusions.
Enteral fluid therapy is typically administered to horses via a nasogastric tube; an indwelling nasogastric tube may be maintained for repeat administration or continuous infusions.
 Small amounts of pure water may be administered enterally, but if large volumes or prolonged enteral fluid therapy is required then an isotonic electrolyte solution should be used to prevent the development of hyponatraemia.
Small amounts of pure water may be administered enterally, but if large volumes or prolonged enteral fluid therapy is required then an isotonic electrolyte solution should be used to prevent the development of hyponatraemia.
 Large volume enteral fluid therapy is rarely used in neonatal foals.
Large volume enteral fluid therapy is rarely used in neonatal foals.
 In foals requiring fluid therapy in which the above routes are not usable, intraosseus fluids may be administered. However, it is difficult to maintain intraosseus catheters for long periods of time, and rapid or large volume fluid administration through this route can be challenging.
In foals requiring fluid therapy in which the above routes are not usable, intraosseus fluids may be administered. However, it is difficult to maintain intraosseus catheters for long periods of time, and rapid or large volume fluid administration through this route can be challenging.
 Subcutaneous fluids are not well tolerated in horses and foals and should be avoided. Similarly, the risks associated with intraperitoneal fluid administration in horses (e.g. gastrointestinal puncture, peritonitis) almost always preclude the use of this method in horses or foals.
Subcutaneous fluids are not well tolerated in horses and foals and should be avoided. Similarly, the risks associated with intraperitoneal fluid administration in horses (e.g. gastrointestinal puncture, peritonitis) almost always preclude the use of this method in horses or foals.
Rate of fluid administration
 Severely dehydrated patients showing signs of shock require rapid intravenous fluid administration, at 40–90 mL/kg/hour, to quickly restore intravascular volume.
Severely dehydrated patients showing signs of shock require rapid intravenous fluid administration, at 40–90 mL/kg/hour, to quickly restore intravascular volume.
 The patient should be closely monitored and the fluid rate modified accordingly as the hydration status improves.
The patient should be closely monitored and the fluid rate modified accordingly as the hydration status improves.
 In adult horses, multiple large bore catheters and fluid pumps may be required to achieve such rapid rates (e.g. ~40 L/hour for a 450 kg horse).
In adult horses, multiple large bore catheters and fluid pumps may be required to achieve such rapid rates (e.g. ~40 L/hour for a 450 kg horse).
 Therefore, hypertonic crystalloids and/or colloids followed by isotonic crystalloids may be alternatively or additionally used in these cases to more rapidly increase intravascular volume.
Therefore, hypertonic crystalloids and/or colloids followed by isotonic crystalloids may be alternatively or additionally used in these cases to more rapidly increase intravascular volume.
 In patients with less severe dehydration or in those receiving maintenance fluids only, the administration rate is determined by dividing the total volume of fluid required in 24 hours into intermittent boluses or a constant rate infusion (CRI). For instance, if the total 24-hour fluid volume required by a 40 kg foal with estimated 5% dehydration is 6 L (2 L hydration deficit and 4 L maintenance needs at 100 mL/kg/day), then the foal could receive a 1L bolus every 4 hours or a CRI of 250 mL/hour. Specialized IV sets that permit calculation of volume per hour based on drip rate or an electronic fluid pump are required for accurate administration of a CRI.
In patients with less severe dehydration or in those receiving maintenance fluids only, the administration rate is determined by dividing the total volume of fluid required in 24 hours into intermittent boluses or a constant rate infusion (CRI). For instance, if the total 24-hour fluid volume required by a 40 kg foal with estimated 5% dehydration is 6 L (2 L hydration deficit and 4 L maintenance needs at 100 mL/kg/day), then the foal could receive a 1L bolus every 4 hours or a CRI of 250 mL/hour. Specialized IV sets that permit calculation of volume per hour based on drip rate or an electronic fluid pump are required for accurate administration of a CRI.
 No more than 6–8 L at one time or 2–4 L/hour of enteral fluids should be administered to an adult horse to avoid gastric distension and associated discomfort.
No more than 6–8 L at one time or 2–4 L/hour of enteral fluids should be administered to an adult horse to avoid gastric distension and associated discomfort.
 Enteral fluid therapy rates should be slowed or discontinued if signs of colic or abdominal distension are observed during or after enteral fluid administration.
Enteral fluid therapy rates should be slowed or discontinued if signs of colic or abdominal distension are observed during or after enteral fluid administration.
Management of common electrolyte and acid–base disturbances
 5% dextrose in water or 0.45% saline/2.5% dextrose are ideal because these fluids are isotonic but provide more free water than other types of fluid once the dextrose is metabolized.
5% dextrose in water or 0.45% saline/2.5% dextrose are ideal because these fluids are isotonic but provide more free water than other types of fluid once the dextrose is metabolized.
Potassium derangements
Other electrolyte derangements
Metabolic acidosis
 HCO3− deficit (mEq) = (25 – patient HCO3−) × 0.3 × body weight (kg).
HCO3− deficit (mEq) = (25 – patient HCO3−) × 0.3 × body weight (kg).
 In foals, use 0.5 instead of 0.3 due to increased extracellular fluid volume.
In foals, use 0.5 instead of 0.3 due to increased extracellular fluid volume.
 Half the calculated deficit should be diluted in an isotonic fluid such as 5% dextrose that does not contain calcium or sodium, and administered as a slow IV bolus over approximately 1 hour. The remainder of the deficit may then be administered in IV fluids (supplemented with potassium – see above) over the next 12–24 hours.
Half the calculated deficit should be diluted in an isotonic fluid such as 5% dextrose that does not contain calcium or sodium, and administered as a slow IV bolus over approximately 1 hour. The remainder of the deficit may then be administered in IV fluids (supplemented with potassium – see above) over the next 12–24 hours.
 In patients with significant ongoing bicarbonate losses, administration of significantly more bicarbonate than the initial deficit is often necessary, so frequent monitoring of electrolyte and acid–base status is vital.
In patients with significant ongoing bicarbonate losses, administration of significantly more bicarbonate than the initial deficit is often necessary, so frequent monitoring of electrolyte and acid–base status is vital.
Metabolic alkalosis
26.2 Pathophysiology and management of shock
Pathophysiology of shock
 Q is the product of stroke volume (SV) and heart rate (HR). SV is affected by the preload and afterload to the heart as well as the heart’s contractility:
Q is the product of stroke volume (SV) and heart rate (HR). SV is affected by the preload and afterload to the heart as well as the heart’s contractility:
 CaO2 is the product of oxygen carried by haemoglobin and dissolved in the blood (PaO2). The amount of oxygen carried by haemoglobin is the product of the amount of haemoglobin in the blood ([Hb]) and the saturation of that haemoglobin with oxygen (SaO2). It is very important to recognize that most of the oxygen in the blood is carried by haemoglobin
CaO2 is the product of oxygen carried by haemoglobin and dissolved in the blood (PaO2). The amount of oxygen carried by haemoglobin is the product of the amount of haemoglobin in the blood ([Hb]) and the saturation of that haemoglobin with oxygen (SaO2). It is very important to recognize that most of the oxygen in the blood is carried by haemoglobin
Classifications and types of shock
 Haemorrhage – middle uterine artery bleed, laceration of a large artery associated with a fracture or wound.
Haemorrhage – middle uterine artery bleed, laceration of a large artery associated with a fracture or wound.
 Fluid loss – diarrhoea associated with colitis, gastric reflux associated with enteritis, sweating with exercise, or fluid leakage associated with extensive burns.
Fluid loss – diarrhoea associated with colitis, gastric reflux associated with enteritis, sweating with exercise, or fluid leakage associated with extensive burns.
 Third space loss – pleural or peritoneal effusions, or fluid trapped within the large colon secondary to a large colon volvulus.
Third space loss – pleural or peritoneal effusions, or fluid trapped within the large colon secondary to a large colon volvulus.
Distributive shock
Stages of shock
 Fluid moves from the interstitial space into the vascular space causing an increase in blood volume.
Fluid moves from the interstitial space into the vascular space causing an increase in blood volume.
 Decreased mean arterial pressure is sensed by baroreceptors (aorta and carotid), triggering catecholamine release, which leads to arterial and venous constriction, increased myocardial contractility, and increased heart rate. It also results in increased antidiuretic hormone (vasopressin) secretion, which stimulates an increase in water resorption in the kidney to increase blood volume.
Decreased mean arterial pressure is sensed by baroreceptors (aorta and carotid), triggering catecholamine release, which leads to arterial and venous constriction, increased myocardial contractility, and increased heart rate. It also results in increased antidiuretic hormone (vasopressin) secretion, which stimulates an increase in water resorption in the kidney to increase blood volume.
 Decreased arterial pressure in the kidney results in activation of the renin-angiotensin-aldosterone pathway, which results in systemic vasoconstriction to increase blood pressure and increased sodium and thus water resorption to increase blood volume.
Decreased arterial pressure in the kidney results in activation of the renin-angiotensin-aldosterone pathway, which results in systemic vasoconstriction to increase blood pressure and increased sodium and thus water resorption to increase blood volume.
 Catecholamine and ACTH release leads to increased circulating cortisol, which stimulates gluconeogenesis and protein catabolism to meet increased cellular energy demands.
Catecholamine and ACTH release leads to increased circulating cortisol, which stimulates gluconeogenesis and protein catabolism to meet increased cellular energy demands.
Management of shock
Therapies directed at restoring cardiac output
 Initially, a bolus of approximately
Initially, a bolus of approximately  of this dose can be given and the status of the patient reassessed.
of this dose can be given and the status of the patient reassessed.
 However, rapid administration of the large volumes required can be difficult in horses. In addition, due to redistribution to the extravascular space, only 25% of the volume administered remains in the intravascular space after 1 hour.
However, rapid administration of the large volumes required can be difficult in horses. In addition, due to redistribution to the extravascular space, only 25% of the volume administered remains in the intravascular space after 1 hour.
 Hypertonic saline solution accomplishes this by drawing fluid out of the interstitium, though this fluid eventually redistributes, resulting in only short-term (<1 hour) volume expansion.
Hypertonic saline solution accomplishes this by drawing fluid out of the interstitium, though this fluid eventually redistributes, resulting in only short-term (<1 hour) volume expansion.
 Thus, to maintain the volume expansion and rehydrate the interstitium, large volumes of isotonic fluids (10 L for every 1 L administered) must be given concurrently with hypertonic saline solution.
Thus, to maintain the volume expansion and rehydrate the interstitium, large volumes of isotonic fluids (10 L for every 1 L administered) must be given concurrently with hypertonic saline solution.
 Colloids are fluids that have high colloid oncotic pressure (COP) due to high-molecular-weight molecules. These molecules remain within the intravascular space to pull and maintain fluid intravascularly, providing more sustained volume expansion compared to crystalloids.
Colloids are fluids that have high colloid oncotic pressure (COP) due to high-molecular-weight molecules. These molecules remain within the intravascular space to pull and maintain fluid intravascularly, providing more sustained volume expansion compared to crystalloids.
 Colloids can be synthetic or natural (e.g. blood products). In general, synthetic colloids have higher COP than natural colloids (with the COP of normal blood), and therefore provide more volume expansion. Additionally, natural colloids carry a risk for transfusion reactions and therefore cannot be administered as rapidly as synthetic colloids. However, some synthetic colloids may interfere with coagulation at high doses.
Colloids can be synthetic or natural (e.g. blood products). In general, synthetic colloids have higher COP than natural colloids (with the COP of normal blood), and therefore provide more volume expansion. Additionally, natural colloids carry a risk for transfusion reactions and therefore cannot be administered as rapidly as synthetic colloids. However, some synthetic colloids may interfere with coagulation at high doses.
Therapies directed at maximizing the oxygen content in the blood
Monitoring
 In properly functioning kidneys, urine production is a crude estimate of blood flow to the kidney.
In properly functioning kidneys, urine production is a crude estimate of blood flow to the kidney.
 Urine specific gravity can also be measured to provide information regarding hydration status and renal function.
Urine specific gravity can also be measured to provide information regarding hydration status and renal function.
 Tissues that are not receiving adequate oxygen turn to anaerobic metabolism to meet energy demands, resulting in hyperlactataemia and acidosis.
Tissues that are not receiving adequate oxygen turn to anaerobic metabolism to meet energy demands, resulting in hyperlactataemia and acidosis.
 Tissues that are not receiving adequate oxygen extract more of the oxygen carried in the blood as it passes through, resulting in a decrease in venous oxygen saturation.
Tissues that are not receiving adequate oxygen extract more of the oxygen carried in the blood as it passes through, resulting in a decrease in venous oxygen saturation.
 The primary determinants of CVP are venous return and cardiac function – volume depletion causes a low CVP, and poor cardiac function results in a high CVP. In standing horses CVP should be between 4 and 12 cm H2O.
The primary determinants of CVP are venous return and cardiac function – volume depletion causes a low CVP, and poor cardiac function results in a high CVP. In standing horses CVP should be between 4 and 12 cm H2O.
 CVP can be helpful in assessing whether adequate volume resuscitation has been performed and in preventing excessive volume expansion, especially in patients with heart disease.
CVP can be helpful in assessing whether adequate volume resuscitation has been performed and in preventing excessive volume expansion, especially in patients with heart disease.
26.3 Administration of blood products in equine critical care
Plasma
Products used in specific circumstances
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine