CHAPTER 46Collection and Transfer of Oocytes in Mares
Methods to successfully collect and transfer equine oocytes have resulted in new reproductive technologies. Because oocytes are collected directly from the follicles, various reproductive pathologic conditions of donors can be avoided, and offspring can be obtained from mares that are considered infertile using standard breeding techniques or embryo transfer.
COLLECTION AND TRANSFER OF THE PREOVULATORY OOCYTE
Commercial Use of Oocyte Transfer
Oocyte transfer has been used to obtain pregnancies from mares that are unsuccessful embryo donors because of various reproductive pathologic conditions.1 Because fertilization, embryo development, and fetal development occur within the recipient, pathologic conditions associated with ovulation or the tubular genitalia of donors are avoided. Although the first foal after an oocyte transfer was obtained in 1988,2 the technique was not used for commercial transfers until the late 1990s.1,3 A number of factors could affect the development or survival of embryos before they are recovered from the uterus between 6 and 8 days after ovulation.
The effects of pathologic conditions associated with ovulation and the oviduct on fertility are not well defined. However, results of studies suggest that reductions in fertility occur before the embryo enters the uterus, especially in older mares. The oviducts of old mares (≥20 years) and young mares (2 to 9 years) were flushed between 1 and 4 days after ovulation. Recovery rates of recently ovulated oocytes or embryos were significantly lower for old compared with young mares (17/29, 59%, and 26/27, 96%, respectively).4 Results of the study suggest that either ovulation does not occur or that the oocyte does not enter the oviduct in some older mares. The incidence of ovulation failure increased with aging in mares and during the autumn months.5,6
In addition to collection and movement of the oocyte, the oviduct functions in the selection and storage of sperm. Scott et al7 compared numbers and motility of sperm in the oviducts of fertile and subfertile mares. Few sperm in the oviducts of subfertile mares were motile, but highly motile sperm were found in the oviducts of normal mares. Oviductal pathologic conditions could be associated with failure of sperm transport and fertilization in subfertile mares. Globular masses, composed of type I collagen, were found more frequently in the oviducts of older than younger mares.8 The effect that these masses have on fertility is not known. Further research is needed to define the extent that follicular and oviductal pathologic conditions affect fertility in the mare.
The Oocyte Donor
For commercial transfers, oocytes are often collected from maturing follicles between 0 and 16 hours before anticipated ovulation. The collection of oocytes from preovulatory follicles has the disadvantage that only one or two oocytes are collected per cycle. However, collection of oocytes from preovulatory follicles has many advantages. The equine cumulus complex in the immature follicle has a broad base of attachment and cellular projections into the theca interna.9 Therefore collection of oocytes from immature follicles, before the luteinizing hormone (LH) surge, is difficult, and collection rates are low. In addition, the maturation in vitro of immature oocytes is more difficult than in vitro culture of preovulatory (maturing) oocytes. Immature oocytes are matured in a complex medium containing additions of hormones and, potentially, growth factors for approximately 30 hours; in contrast, culture of preovulatory oocytes can be accomplished in a relatively simple medium within 12 hours. After maturation, competence of oocytes matured in vitro is probably reduced when compared with oocytes collected from the preovulatory follicle. Therefore, in this author’s laboratory, oocytes for commercial transfers are usually collected within 16 hours before the anticipated ovulation of the dominant follicle.
Requirements for oocyte donors are minimal. The donor needs to grow a preovulatory follicle containing a viable oocyte. In general, oocytes from young donors (3 to 13 years) are considered more viable than oocytes from older donors. When oocytes were collected from the preovulatory follicles of young donors (6 to 10 years) and old donors (20 to 26 years) and transferred into the oviducts of young recipients (3 to 7 years), significantly more oocytes from young compared with old donors developed into embryonic vesicles (11/12, 92%, versus 8/26, 31%).10 Oocytes from old versus young mares had more morphologic anomalies, such as large vesicles or irregular shapes, when imaged using light and electron microscopy.11 However, pregnancies were obtained from old (≥20 years) mares in this author’s commercial oocyte transfer programs,1 although more transfers were required to produce a pregnancy when oocytes were collected from older mares.
Oocyte Collection
Oocytes have been collected from the follicles of mares using laparotomies,12 colpotomies,13,14 flank punctures,15 and transvaginal, ultrasound-guided, follicular aspirations.16,17 In this author’s laboratory, oocytes are usually collected by ultrasound-guided punctures through the vaginal wall. For some mares, aspirations through the vagina can be difficult because of pathologic conditions such as melanomas or severe uterine discharge. In these instances we use flank punctures to collect oocytes.
Transvaginal, ultrasound-guided follicular aspirations require use of an ultrasound machine and a transducer casing with a needle guide (Figure 46-1). Linear, curvilinear, and sector transducers have been used. The rectum is evacuated, and the vulva is cleaned. The mare is sedated and twitched. Rectal contractions have been minimized through the administration of propantheline bromide (0.04 mg/kg IV)1 or the intrarectal deposition of lidocaine. A nontoxic lubricant is applied to the transducer to improve contact with the vaginal wall. The transducer is positioned within the anterior vagina, lateral to the posterior cervix and ipsilateral to the preovulatory follicle. Transrectal manipulations are used to position the follicle with the follicular apex juxtaposed to the needle guide. A needle is advanced through the needle guide to puncture the vaginal and follicular walls. In the author’s laboratory, a 12-gauge, double-lumen needle is used (Cook Veterinary Products, New Buffalo, Mich.). The follicular fluid is aspirated from the follicle using a pump (Cook Veterinary Products, New Buffalo, Mich.) set at 150 mm Hg. As the follicular fluid is aspirated from the follicle, the lumen is lavaged with between 50 and 100 ml of flush, typically modified Dulbecco’s Phosphate buffered solution or an embryo flush solution (emCare, ICP, Auckland, New Zealand) with additives of fetal calf serum (1%) or bovine serum albumin (0.4%) and heparin (10 IU/ml).
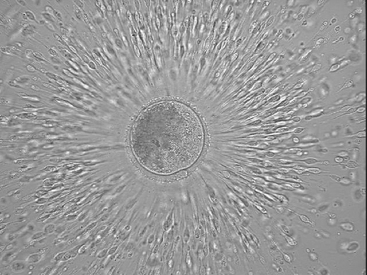
The equine oocyte is slightly greater than 100 µm in diameter; however, it is surrounded by a mass of cumulus cells that is often greater than 1 mm in diameter (Figures 46-2 and 46-3). The cumulus complex appears translucent in the bloody flush solution and usually can be seen with the naked eye. After identification, the cumulus oocyte complex should be washed in medium to remove attached blood and debris.
Oocyte Culture and Transfer
Different scenarios can be used for timing oocyte collections and transfers. However, the complete interval between administration of hCG and transfer is usually between 36 and 40 hours. In one study the time of oocyte collection (24 versus 36 hours after administration of hCG to donors) did not affect pregnancy rates.18 Similar results have been obtained in this author’s laboratory, with good pregnancy rates for oocytes collected at different intervals after administration of hCG.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree