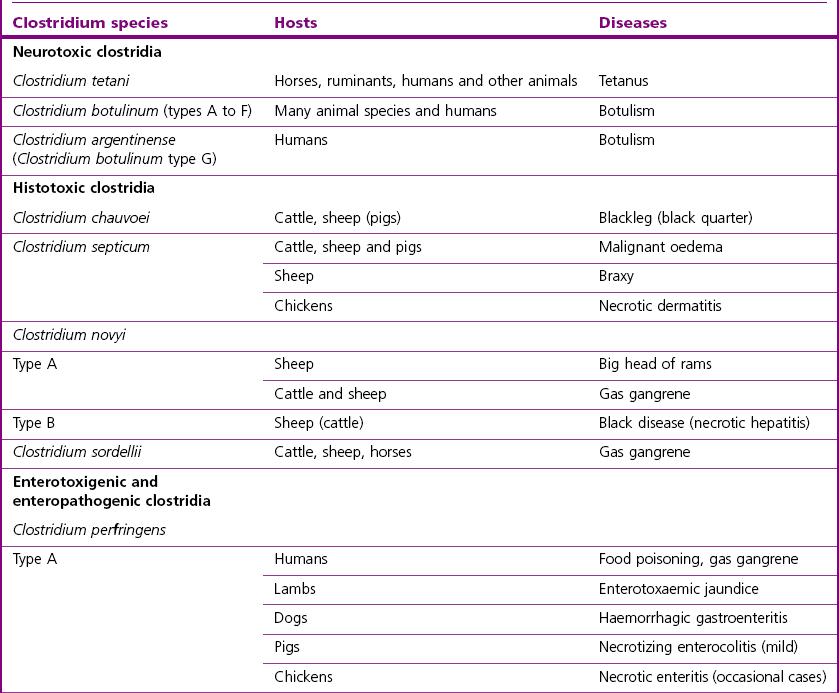
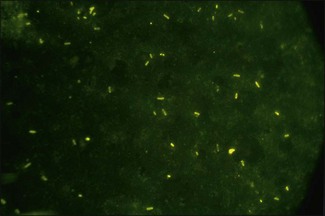
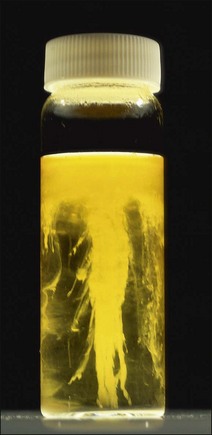
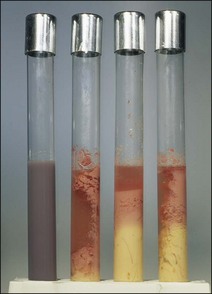
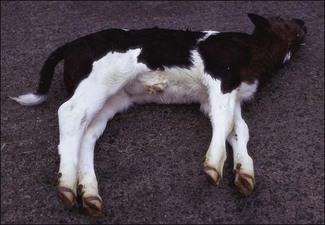
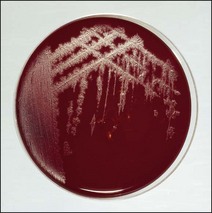
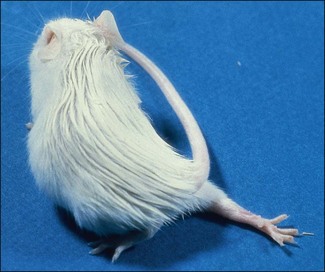
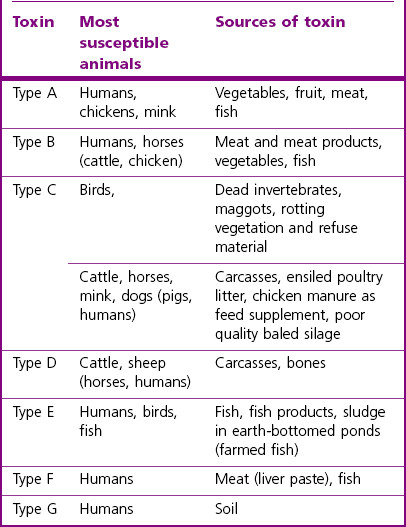
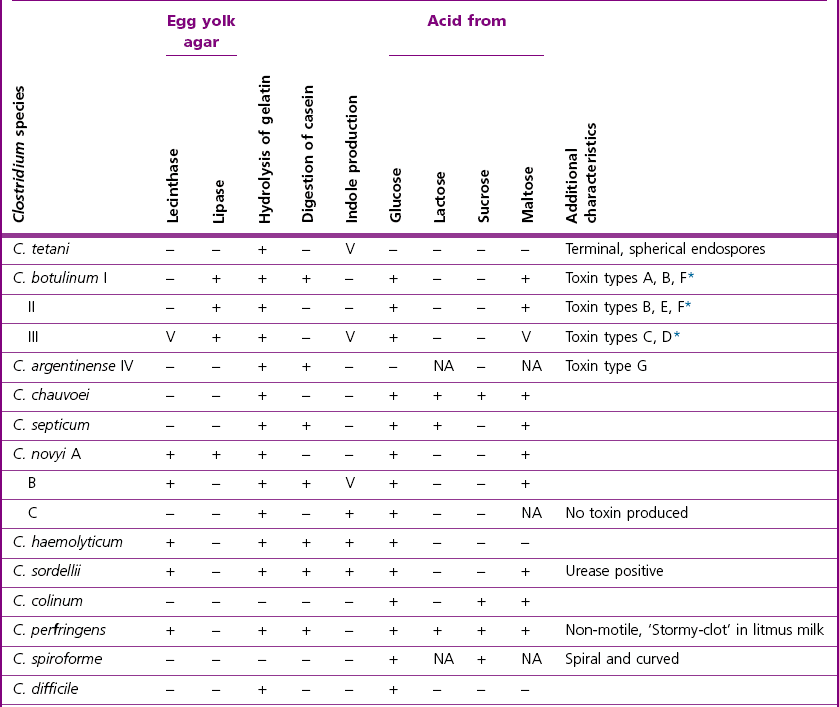
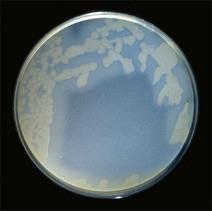
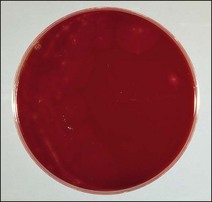
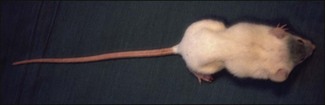
Chapter 16 • Neurotropic clostridia (C. tetani and C. botulinum) that produce potent neurotoxins but are non-invasive and colonize the host to a very limited extent. • Histotoxic clostridia (C. chauvoei, C. septicum, C. novyi types A and B, C. haemolyticum, C. sordellii and C. perfringens type A) that produce less potent toxins than the first group but are invasive. This includes the gas-gangrene-producing clostridia. • Clostridia that are enteropathogenic or produce enterotoxaemias (C. perfringens types A-E, C. difficile, C. colinum and C. spiroforme). Enterotoxins are formed in the intestines and absorbed into the blood stream producing a generalized toxaemia. • The atypical clostridium, C. piliforme, which causes Tyzzer’s disease in foals and laboratory animals. Table 16.1 summarizes the hosts and diseases of the pathogenic clostridia. • Gram-stained smears from affected tissues may reveal large Gram-positive rods that tend to decolourize easily when sporing. Clostridium spiroforme is an exception being curved or helical. The characteristic ‘drumstick’ forms of C. tetani, (Fig. 16.1) due to the spherical spores being terminal and bulging the cell, may be seen in necrotic material from wounds associated with tetanus. This is suggestive, but by no means conclusive, as other clostridia, such as C. tetanomorphum have a similar morphology. In cases of suspected enterotoxaemia, the presence of large numbers of fat Gram-positive rods in a smear of the small intestinal mucosa, from a recently dead animal (Fig. 16.2), is presumptive evidence of the condition. Figure 16.1 Sporing rods of Clostridium tetani in Gram-stained smear of necrotic material from a penetrating wound. The spores are spherical, terminal and bulge the mother cell giving the typical ‘drum-stick’ appearance. Figure 16.2 Clostridium perfringens: large Gram-positive rods in a mucosal scraping from the small intestine of a lamb that had recently died from pulpy kidney disease. (Gram stain, ×1000) • Fluorescent antibody (FA) technique is used routinely for diseases associated with C. chauvoei (Fig. 16.3), C. septicum, C. novyi and C. sordellii as fluorescent labelled antisera can be obtained commercially. Affected tissue as well as a piece of rib containing bone marrow (about 14 cm long) are useful specimens. A bacteraemia usually occurs with these clostridial diseases so the bacteria would be expected to be present in bone marrow. This tissue has the added advantages of giving low background autofluorescence and being one of the last tissues to be invaded, postmortem, by bacteria such as C. septicum. In general, freshly prepared or pre-reduced blood agar is suitable for the isolation of clostridia. Media for the more fastidious anaerobes such as C. chauvoei, C. haemolyticum and C. novyi types B and C are given in Appendix 2. Stored agar media gradually absorb oxygen from the atmosphere, so it is important to use either freshly prepared blood agar or pre-reduced plates that have been stored under anaerobic conditions soon after preparation. A blood agar and a MacConkey agar plate should be inoculated and incubated aerobically. These plates will detect any aerobic pathogens that may be present and also indicate the degree of contamination of the specimen by facultative anaerobes. Liquid and semisolid media with a low redox potential such as cooked meat broth and thioglycollate medium (Fig. 16.4) can be used to grow and maintain pure cultures of the clostridia. They are of limited use for primary inoculation as any fast-growing anaerobes or facultative anaerobes will outgrow the Clostridium species of interest. Immediately before inoculating cooked meat broth or thioglycollate medium, they should be boiled to expel absorbed oxygen and then rapidly cooled to 37°C. Some of the biochemical reactions of the pathogenic clostridia are given in Table 16.2. On egg yolk agar the clostridia with lecithinase activity produce an opalescent change around the colonies due to enzymatic action on the lecithin in the medium. Those producing a lipase cause a pearly layer or iridescent film that can cover the colonies and in some cases extend into the surrounding agar (Fig. 16.5). Clostridium perfringens inoculated into litmus milk medium produces the classical ‘stormy-clot’ or ‘stormy-fermentation’ reaction (Fig. 16.6). The lactose in the medium is fermented by C. perfringens producing acid which coagulates the casein and induces a colour change from blue to pink (litmus pH indicator). The acid clot is then broken up by gas formation. Miniaturized commercial systems such as API 20A (bioMérieux) or Rapid ID 32A (bioMérieux) are available for the identification of many of the clostridia. The principal fermentation products can also be used to identify the Clostridium species by gas chromatography. Table 16.2 Biochemical reactions of the clostridia pathogenic for animals + = positive reaction, − = negative reaction, V = variable reaction, NA = data not available Figure 16.5 Clostridium botulinum type C on egg yolk medium giving a pearly layer around the colonies due to lipase activity. Lecithinase is not produced by this bacterium. • As ‘biological filters’ for contaminated specimens or for material judged to contain small numbers of the pathogenic Clostridium species. • In neutralization or protection tests to specifically identify the toxin(s) present and hence the clostridial pathogen involved in the disease. These procedures are most commonly used in tetanus, botulism and in the enterotoxaemias caused by C. perfringens. Clostridium tetani is a straight, slender (0.4–0.6 × 2–5 µm), Gram-positive rod that characteristically produces a terminal, spherical endospore that bulges the cell giving the characteristic ‘drumstick’ appearance to the bacterium (Fig. 16.1). The endospores are highly resistant and although boiling kills the spores of most strains in 15 minutes, autoclaving at 121°C for 15 minutes is completely sporicidal. There are 10 serological types of C. tetani, based on flagellar antigens, but the neurotoxin is antigenically uniform. Clostridium tetani produces the exotoxins tetanolysin (a haemolysin) which may enhance tissue invasion and tetanospasmin (a neurotoxin) which is plasmid-coded and responsible for the signs of tetanus. The endospores enter traumatic or surgical wounds, especially after castration or docking, via the neonatal umbilicus (Fig. 16.7) or into the uterus following dystocia in cattle and sheep. The presence of facultative anaerobes and necrotic tissue create anaerobic conditions and the C. tetani spores germinate. The vegetative cells multiply at the entry site and produce the potent tetanospasmin. This travels via peripheral nerves or blood stream to ganglioside receptors of the motor nerve terminals to which it binds irreversibly. The toxin travels to the nerve cell body and its dendritic processes in the central nervous system by retrograde intra-axonal flow. The toxin then undergoes transcytosis from the motor neuron to its site of action in the inhibitory neurons. Tetanus toxin is a bipartite toxin with the light chain being the toxic moiety. The heavy chain mediates attachment and internalization of the toxin. Following internalization which takes place through endocytosis, the low pH of the endosome induces a conformational change in the heavy chain, causing it to form a pore through which the light chain translocates into the cytosol. The disulphide bridge joining the two chains is reduced in the cytosol. The light chain is a zinc endopeptidase which cleaves synaptobrevin, a vesicle-associated membrane protein. Cleavage of this protein prevents the vesicles containing inhibitory neurotransmitters from releasing their contents, resulting in spastic paralysis and the characteristic tetanic spasms. Tetanospasmin binds specifically to gangliosides in nerve tissue and once bound cannot be neutralized by antitoxin. When toxin travels up a regional motor nerve in a limb, tetanus develops first in the muscles of that limb, then spreads to the opposite limb and moves upwards. This is known as ascending tetanus and is usually seen only in the less susceptible animals such as dogs and cats. Descending tetanus is the common form in susceptible species such as humans and horses. In this form toxin circulating in the blood stream affects the susceptible motor nerve centres that serve the head and neck first and later the limbs. Once established, signs of tetanus are similar in all animal species. In tetanus the diagnosis is often based on the history and on the characteristic clinical signs. Gram-stained smears of material from a wound may reveal the characteristic ‘drumstick’ sporing forms of C. tetani (Fig. 16.1). This is not completely diagnostic as other clostridia such as C. tetanomorphum have a similar morphology. Clostridium tetani is haemolytic and on normal blood agar tends to have a spreading, swarming growth (Figs 16.8 and 16.9) while on ‘stiff agar’ (3%) individual rhizoid colonies are formed (Fig. 16.10). Figure 16.8 Clostridium tetani colonies on sheep blood agar showing spreading growth and a narrow zone of beta-haemolysis. Figure 16.9 Spot inoculation of C. tetani to illustrate the characteristic spreading growth on normal blood agar containing 1.5% agar. Oblique illumination. Clostridium tetani liquefies gelatin but does not ferment the usual range of carbohydrates. Other reactions are given in Table 16.2. Alternatively, PCR-based procedures can be used to identify C. tetani colonies (Akbulut et al. 2005). Demonstration and identification of the toxin is more important than the isolation and identification of the bacterium. The toxin present in an animal’s serum or in filtrate from cooked meat broth or thioglycollate medium, can be demonstrated in laboratory animals and identified by neutralization or protection tests using specific antitoxin. In the protection test the animals are protected with antitoxin at least two hours before inoculation with the material containing toxin. The control mice show typical signs of tetanic spasm in the region of inoculation (Fig. 16. 11). Clostridium botulinum is a straight rod (0.9–1.2 × 4–6 µm) and at a pH near or above neutrality produces oval, subterminal spores. The spores are very resistant but are killed at 121°C for 15 minutes while the toxins are destroyed at 100°C for 20 minutes. Four phenotypically distinct groups of C. botulinum are recognized (Sharma & Whiting 2005) as shown in Table 16.2. In addition, seven types of C. botulinum are distinguishable based on the antigenicity of the toxin produced. Eight different neurotoxins are produced by C. botulinum types A–G (two types of C toxin). Clostridium botulinum Type G has been renamed C. argentinense. The toxins are identical in action but differ in potency, distribution and antigenicity and those of types C and D are known to be bacteriophage-coded. The optimum pH for C. botulinum is neutral to slightly alkaline (pH 7.0–7.6) and the optimal temperature lies between 30–37°C. The endospores are widely, but unevenly, distributed in soils and aquatic environments throughout the world. Germination of the endospores, with growth of vegetative cells and production of toxin, occurs in anaerobic situations such as contaminated cans of meat, fish or vegetables, carcasses of invertebrate and vertebrate animals, rotting vegetation and baled silage. Table 16.3 indicates the toxins produced, source of toxin and animals susceptible to each of the C. botulinum types. Botulism is an intoxication usually caused by ingestion of preformed toxin in foodstuffs. The toxin is absorbed from the intestinal tract and is transported via the bloodstream to peripheral nerve cells where it acts at the neuromuscular junctions of cholinergic nerves and also at peripheral autonomic synapses. Botulinum toxin has a similar structure and mode of action to tetanus toxin with differences in clinical signs in the two diseases attributable to the different sites of action of the toxins. The heavy chain binds to susceptible cells and the toxin enters the cell through endocytosis. Following entry into the cytosol the zinc metalloprotease acts on synaptobrevin and other SNARE (SNAP (Soluble NSF Attachment Protein) Receptor) proteins which prevent the release of acetylcholine at the myoneural junctions. This results in flaccid paralysis, death being caused by circulatory failure and respiratory paralysis. Less common methods of acquisition of toxin are wound botulism (toxicoinfection) and infant botulism (intraintestinal toxicoinfection). In wound botulism the spores are introduced into wounds where they germinate. Toxin is formed at this localized site and spreads through the body. The ‘shaker foal’ syndrome is thought to be caused in this way. In humans, wound botulism is increasingly seen in drug addicts following use of contaminated needles. Infant botulism occurs when spores germinate in the intestines when the normal flora has not yet been fully established. This form is seen in human infants (‘floppy baby’ syndrome) and as rare epidemics of type C in broiler chickens and turkey poults. The toxin is one of the most potent known: one milligram of the neurotoxin contains more than 120 million mouse lethal doses. A comparison of the toxins of C. tetani and C. botulinum is shown in Table 16.4. Botulism is most common in water birds (Fig. 16.12), ruminants, horses, mink and poultry. Carnivores are relatively resistant to all types and pigs are susceptible to the toxin of type A but resistant to those of B, C and D. The oral toxicity of type D toxin is high for cattle and type C toxins are more readily absorbed through the intestinal wall of chickens and pheasants. Table 16.4 Comparison of the toxins of Clostridium tetani and Clostridium botulinum Serum or centrifuged serous exudates from animals can be directly inoculated intraperitoneally (0.5 mL) into mice. If toxin is present the characteristic ‘wasp waist’ appearance (Fig. 16.13) in the mice will be seen in a few hours or up to five days. The appearance is due to abdominal breathing because of paralysis of respiratory muscles. Cattle are extremely susceptible to botulism and detection of toxin in serum is difficult. Thus detection of toxin in gastrointestinal contents, which have been frozen immediately after collection to prevent postmortem multiplication of C. botulinum organisms, may be a more rewarding approach (Hogg et al. 2008). Figure 16.13 Mouse inoculated with serum containing the toxin of C. botulinum. Note the characteristic ‘wasp-waist’ appearance. ELISA can be used for the detection of toxin but none of the currently available assays are as sensitive as mouse bioassay (Cai & Singh 2007).
Clostridium species
Pathogenicity
Laboratory Diagnosis (General)
Direct microscopy
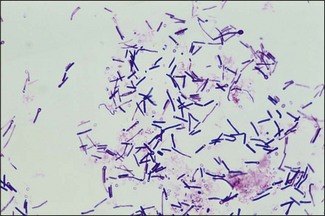
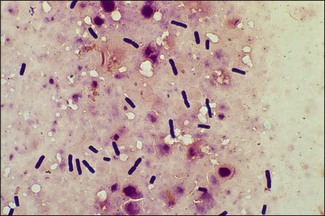
General isolation procedures
Biochemical reactions
Animal inoculation
Neurotoxic clostridia
Clostridium tetani
Pathogenesis
Laboratory diagnosis
Direct microscopy
Identification
Colonial morphology

Biochemical reactions
Toxin identification
Clostridium botulinum
Natural habitat
Pathogenesis
Clostridium tetani
Clostridium botulinum
Site of toxin production
Wounds
Carcasses, decaying vegetation and occasionally wounds and intestine
Location of encoding genes
Plasmid
Chromosome, phage (types C and D)
Structure of toxin
Bipartite, heavy chain (involved in binding), light chain is a protease (toxic moiety)
Bipartite, heavy chain (involved in binding), light chain is a protease (toxic moiety)
Mode of action
Toxin acts centrally.
Cleaves proteins that mediate fusion of neurotransmitter vesicles with presynaptic membrane of inhibitory interneurons
Toxin acts peripherally. Cleaves proteins that mediate fusion of neurotransmitter vesicles with presynaptic membrane of cholinergic nerves
Type of paralysis
Spastic
Flaccid
Antigenic types of toxin
Tetanospasmin (one antigenic type)
Eight different toxins produced by types A to G
Laboratory diagnosis
Toxin demonstration
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clostridium species
Only gold members can continue reading. Log In or Register to continue