CHAPTER 66 Clinical Use of Erythropoietin in Feline Medicine
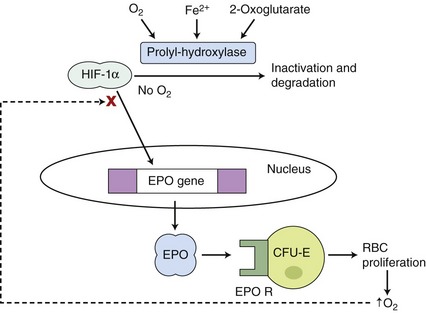
Erythropoietin (EPO) is the hematopoietic growth factor responsible for stimulating erythrogenesis in response to anemia. EPO is a 30.4 kilodalton glycoprotein characterized by a 165–amino acid protein backbone with 4 carbohydrate chains, each containing two to four sialic acid residues.1 The majority of EPO is produced in the peritubular interstitial cells of the inner renal cortex and outer renal medulla; however, the liver produces 10 to 15 per cent of the total EPO.2 EPO has a circulating half-life of 6 to 10 hours. The main trigger for the synthesis of EPO is renal hypoxemia, which increases production of hypoxia-inducible factor (HIF). HIF stimulates transcription of the EPO gene and is inactivated by HIF-alpha prolyl and asparaginyl hydroxylases. HIF-alpha hydroxylases require oxygen and iron to function (Figure 66-1).3
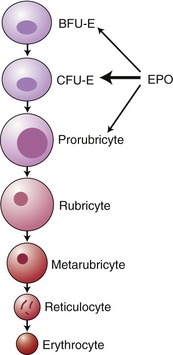
EPO stimulates proliferation and maturation of erythrocytes in the bone marrow directly by binding specific growth factor receptors on the red blood cell precursors. EPO has the greatest effect on the colony-forming unit erythroid cell (CFU-E), although it has some effect also on the differentiation of the burst-forming unit erythroid cell (BFU-E) and erythroblasts (Figure 66-2).4 In the absence of EPO, these progenitor cells undergo apoptosis, whereas they differentiate and proliferate in the presence of EPO.5 It takes approximately 5 days from stimulation by EPO for the release of reticulocytes into the circulation.6
In addition to its effects on red blood cell differentiation, EPO induces synthesis of hemoglobin and red blood cell membrane proteins. It facilitates release of reticulocytes into the blood by lowering the barrier between the marrow and the blood.7
ERYTHROPOIESIS-STIMULATING AGENTS (ESA)
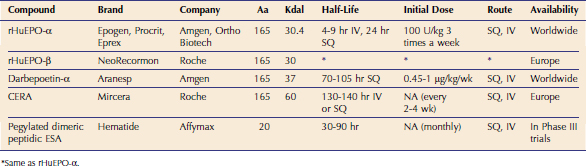
The gene sequence for EPO was determined in 1983, and recombinant human EPO (rHuEPO) became available in 1985.8 Epoetin alfa varies from epoetin beta in the glycosylation, but there is no difference in clinical efficacy.9 These products have a half-life of 4 to 9 hours after IV injection and 24 hours after SQ injection (Table 66-1).5 Darbepoetin was licensed for use in human beings in 2001. Darbepoetin has a 165–amino acid protein backbone that varies from the human EPO sequence by 5 amino acids and is more highly glycosylated than epoetin alfa and beta. The glycosylation slows clearance of this product, allowing a reduction in frequency of administration while maintaining efficacy.10–12
Feline EPO has a greater amino acid sequence homology with human EPO (83.3 per cent) than the canine amino acid sequence (81.3 per cent).13,14 Although the gene sequence is adequately conserved for human products to have a clinical effect in cats and dogs, the absence of complete homology results in the possibility of antibody formation that leads to development of pure red cell aplasia (PRCA) in both species.
Recombinant feline EPO (rFeEPO) and recombinant canine EPO have been produced but are not available commercially.13 In a small number of cats with anemia of chronic kidney disease (CKD) treated with rFeEPO, hematocrit increased in the majority of patients. Unfortunately, a small number of treated cats, both those who had never received rHuEPO and those receiving rFeEPO as a salvage treatment after development of PRCA from rHuEPO, developed PRCA in response to rFeEPO. The reasons for this adverse complication are under investigation, but proposed theories include gene polymorphism in the population or use of an aberrant gene in the initial gene sequencing.15 Recombinant canine EPO has been shown to stimulate erythrocyte production successfully in clinically normal dogs and dogs with CKD without causing PRCA, a complication that was encountered in rHuEPO-treated dogs.16,17 Canine EPO was not uniformly effective in increasing hematocrit in dogs with PRCA induced by rHuEPO.17
The gene for feline EPO has been transfected into an adenovirus vector and introduced into healthy cats via IM injection. Gene transcription with attendant increase in hematocrit was present in the high-dose group. Some cats developed polycythemia, and surgical excision of the injected muscle controlled polycythemia in some of the affected animals. However, some cats continued to produce excessive EPO, and virus particles could be found in surrounding tissue. One cat developed PRCA, which resolved after excision of the injected muscle.18,19 In order to control gene expression, a doxycycline response gene has been inserted and may make this technology feasible in the future.20
Continuous EPO receptor activator (CERA) has the same protein sequence as EPO and has a large carbohydrate chain attached to the protein backbone, doubling its molecular weight compared to EPO.3 Because of its long half-life, the sustained effect on stimulating the EPO receptor increases erythropoiesis21 (see Table 66-1). Initial reports projected that it would need to be administered monthly. Although CERA was approved by the FDA for use in human patients in 2007, it is not yet available commercially, and there is no information about its use in cats or dogs.
A small peptide of 20 amino acids (Hematide) unrelated in sequence to EPO that can stimulate the EPO receptor is in Phase III clinical trials in people (see Table 66-1). Once-monthly IV or SQ injections are effective in treating anemia in human patients.3 This drug is dissimilar to epoetin or native EPO, and therefore it holds promise as a rescue agent for patients with PRCA because it is not inactivated by anti-EPO antibodies. This concept has been shown in mice, and this drug has been used for this purpose in human beings.3,22 When this drug becomes available, its use could be considered for treatment of PRCA in cats.
BENEFITS OF TREATMENT OF ANEMIA
Treating anemia has many benefits, including improving tissue oxygenation, which enhances exercise capacity, central nervous system function, and endocrine and cardiac function.23 In people, control of anemia decreases the number of hospital admissions and reduces cardiovascular morbidity.24 Treatment of anemia improves cognitive function, decreasing depression and increasing quality of life.25 Although these factors are difficult to quantify in animals, owners volunteer comments frequently about the improvement in attitude and social behaviors of their companion animals. Interestingly, this improvement may happen at initiation of rHuEPO treatment, prior to any substantial increase in hematocrit.17,26
Treatment of anemia may slow the progression of renal disease in human beings.28 Hypoxia increases extracellular matrix formation and interstitial fibrosis, and treatment of anemia decreases renal cellular hypoxia. In a randomized clinical trial in human patients, early treatment of anemia (hemoglobin 9 to 11.5 g/dL) decreased the risk of doubling of creatinine, starting dialysis, or death by 60 per cent, compared to delayed treatment of anemia (hemoglobin < 9 g/dL).29 It is interesting to speculate on the effect of early treatment of anemia in veterinary renal patients. Unfortunately, the currently available treatments (mainly rHuEPO) carry the risk of substantial adverse effects, making a clinical trial of early treatment (prior to onset of clinical signs of anemia) difficult to design ethically. New ESAs with lower risk may obviate this dilemma.
INDICATIONS FOR ERYTHROPOIESIS-STIMULATING AGENTS
Human recombinant EPO is approved for use in human beings to treat anemia associated with hemodialysis or chronic kidney disease, cancer patients on chemotherapy, human immunodeficiency virus (HIV) patients on zidovudine (AZT) therapy, and for anemic patients preparing for major surgery who wish to minimize allogeneic blood transfusions. rHuEPO and darbepoetin are used primarily to treat anemia of CKD in cats and dogs but also have been used to treat feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) infections, idiopathic PRCA in cats, myelodysplastic and myelofibrotic disorders, and for autologous transfusions in dogs.30–38
ANEMIA OF CHRONIC KIDNEY DISEASE
Anemia is a common abnormality noted in patients with CKD, affecting 32 to 65 per cent of cats with CKD.39–41 The mechanism of development of anemia of CKD is multifactorial. Reasons for increased loss of erythrocytes include bleeding from gastrointestinal ulceration and uremic platelet dysfunction and decreased survival in the uremic environment.42–44 Decreased production may result from uremic toxins that inhibit erythropoiesis,1,42,44–47 nutritional deficiencies (e.g., B vitamins),45,48,49 bone marrow fibrosis,43,45,50–52 and concurrent inflammation.
The most significant reason for development of anemia associated with CKD is a relative EPO deficiency. Several studies have demonstrated that EPO levels in uremic anemic patients are within the normal range or mildly elevated, in contrast to nonuremic anemic patients, who have markedly elevated EPO levels.43,46,53,54 As the kidneys fail, EPO production decreases, the bone marrow becomes less proliferative, and a slowly developing nonregenerative anemia develops. This relative EPO deficiency can be reversed by treatment with rHuEPO.
Many therapies have been suggested for treatment of anemia of CKD, including minimizing blood loss from gastric ulcers, controlling blood sampling, and administering blood transfusions. Supplementation of the water-soluble B vitamins may be beneficial in CKD patients.55 Anabolic steroids have been utilized for the treatment of the anemia of CKD. Results have been variable and generally disappointing, with a long delay to effect and the effect being minor. Their use can not be recommended due to the lack of efficacy and the potential for hepatotoxicity.48,55,56
Epoetin and darbepoetin have been used successfully for treatment of anemia of CKD in human beings, cats, and dogs. rHuEPO increases the hematocrit effectively in a dose-dependent manner and has been used to treat naturally occurring CKD in cats and dogs.16,27,57,58 In addition to resolving anemia, the majority of patients treated with rHuEPO have improved appetite, weight gain, increased energy, alertness, playfulness, and physical strength, greater affection, and improved grooming.*
CANCER PATIENTS ON CHEMOTHERAPY
Anemia in cancer patients often is multifactorial. The most common mechanisms include anemia of chronic disease, blood loss, decreased production from bone marrow infiltration, and suppression secondary to chemotherapy. Cancer patients may have an inadequate EPO response to an anemia (relative EPO deficiency).61 Erythropoiesis-stimulating agents have been used in human cancer patients in an effort to minimize the need for blood transfusions. Although the improvement in quality of life from resolution of anemia is unquestioned, concern exists about the effect of ESAs on tumor progression and patient survival times.62 EPO may improve sensitivity to radiation therapy and chemotherapy by increasing tumor oxygenation, but it also promotes angiogenesis, which may support tumor growth.5,63 There are no reports of the use of rHuEPO in veterinary cancer patients.
MYELODYSPLASTIC DISORDERS
Myelodysplastic syndrome (MDS) refers to myeloproliferative disorders associated with anemia, neutropenia, and/or thrombocytopenia with the presence of less than 30 per cent dysplastic hematopoietic precursor (blast) cells in the bone marrow (see Chapter 64). rHuEPO has been used successfully to treat human beings, cats, and dogs with MDS. Approximately 25 per cent of human patients with MDS respond to rHuEPO treatment.64 Response to rHuEPO in four cats with PRCA not induced by rHuEPO administration was difficult to determine due to concurrent immunosuppressive therapy but appeared to be of no benefit in two cats.33 There have been reports of successful treatment of myelodysplasia in dogs with rHuEPO in combination with immunosuppressive therapy.34,35 rHuEPO also has been used (in combination with immunosuppressive therapy) for the treatment of myelofibrosis in dogs.37,65
INTENSIVE CARE UNIT PATIENTS
Relative EPO deficiency has been well documented in critically ill patients.66 Impaired iron availability may play a significant role in anemia associated with critical illness.67 rHuEPO treatment appears to be most useful in critically ill patients who have a protracted ICU stay, which corresponds to a limited population in veterinary medicine.
VIRAL IMMUNOSUPPRESSIVE DISEASES
rHuEPO is indicated for treatment of anemic HIV-infected people receiving the antiviral agent zidovudine (AZT) who have low endogenous serum EPO levels. Of the 60 to 80 per cent of HIV patients on zidovudine who had low EPO levels, rHuEPO treatment reduced transfusion requirements by 43 per cent, and 17 per cent were transfusion-independent.68,69 Transfusion requirements did not change in patients with endogenous EPO levels greater than 500 mU/mL. rHuEPO has been suggested as a treatment for anemic cats with FeLV infection, although many patients do not respond.70 Serum EPO levels are elevated in anemic cats infected with FeLV,30,31 so exogenous EPO would not be predicted to be helpful. A short course (2 weeks) of rHuEPO was effective at raising hematocrit in nonanemic FIV-infected cats and did not result in anti-rHuEPO antibody formation.71
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree