CHAPTER 6 Embryo cleavage and blastulation
Upon fertilization, meiosis is completed and cell cyclicity returns to the mitotic pattern. The unique embryonic genome has been established through the mixing of the maternal and paternal chromosomes by dissolution of the two pronuclei. This equips the zygote with the full genetic make-up for building the embryo. The cytoplasm of the zygote, inherited from the oocyte, contains the complete molecular and structural composition necessary to initiate the first cleavages and, later, activate the embryonic genome for de novo embryonic transcription. Hence, the initial phase of development is driven by information stored in the oocyte and passed on to the zygote and the early embryo.
CLEAVAGES AND GENOME ACTIVATION
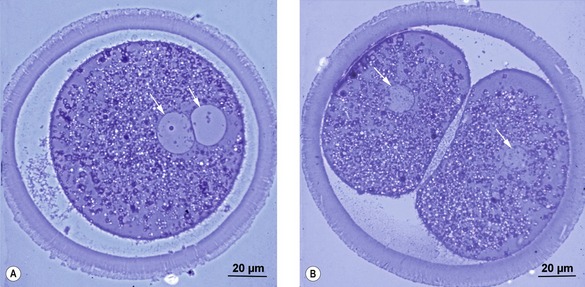
In the zygote, an S-phase is completed during the first post-fertilization cell cycle. Thus, when the zygote cleaves to the 2-cell embryo at the first mitosis, each of the two cells, referred to as blastomeres, obtains its full copy of the embryonic genome (Figs. 2-1, 6-1). The embryo is still surrounded by the zona pellucida and remains so for some days. A number of mitotic divisions follow. During this phase of development, the mitotic divisions are special in that they occur almost without cellular growth; the cells become smaller and smaller as the original cytoplasm of the zygote is divided into smaller and smaller portions. These cell divisions are referred to as cleavages. The blastomeres may be of unequal sizes because of asynchrony in cleavage. This asynchrony becomes apparent from the outset; even between the 2- and the 4-cell stages, it results in temporary 3-cell embryos. At least in the mouse, the point of sperm entry may position the plane of the first cleavage division. Furthermore, the 2-cell-stage blastomere inheriting the sperm entry site tends to divide earlier than the other and is more likely to give rise to cells that become positioned internally in the growing ball of cells.
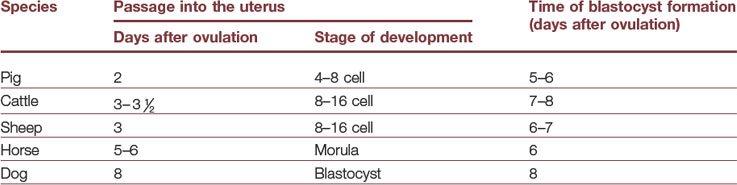
Cleavages begin during the transport of the embryo through the oviduct but, at a species-specific stage of development, the embryo enters the uterus (Table 6-1). The mare is very unusual with regard to passage through the oviduct; only embryos are allowed to enter the uterus while unfertilized oocytes, by an unknown mechanism, are retained in the oviduct.
Table 6-1: Times of passage of the embryo from the oviduct into the uterus, and of blastocyst formation, in different species
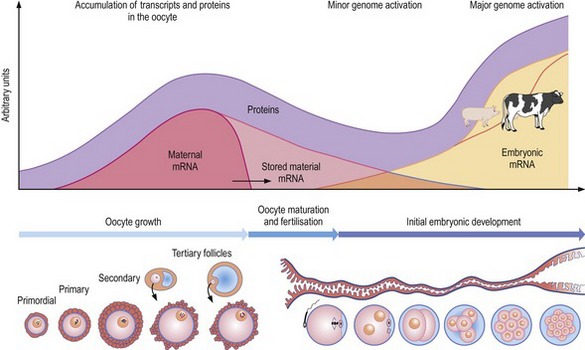
During the growth of the oocyte, transcripts and proteins are stored in this specialized cell for later use (Fig. 6-2). At the end of the growth phase transcription diminishes. However, by then the oocyte is more or less loaded with the transcripts and proteins required for driving initial embryonic development and these govern at least the first cleavage. The transcripts and proteins are gradually degraded after fertilization and, at a certain stage of development, activity of the embryonic genome is required. The embryonic genome is activated gradually; transcription is very limited initially but increases later, at species-specific stages, in two phases – those of minor and major activation of the embryonic genome (Table 6-2).
Table 6-2: Timing of the minor and major activation of the embryonic genome in different species
| Species | Minor genome activation | Major genome activation |
|---|---|---|
| Mouse | G2 of first cell cycle (zygote) | Second cell cycle (2-cell embryo) |
| Pig | Unknown | Third cell cycle (4-cell embryo) |
| Cattle | First cell cycle (zygote) | Fourth cell cycle (8-cell embryo) |
| Dog | Unknown | Fourth cell cycle (8-cell embryo) |
| Horse | Unknown | Fourth to fifth cell cycle (8–16-cell embryo) |
| Sheep | Unknown | Fourth to fifth cell cycle (8–16-cell embryo) |
COMPACTION
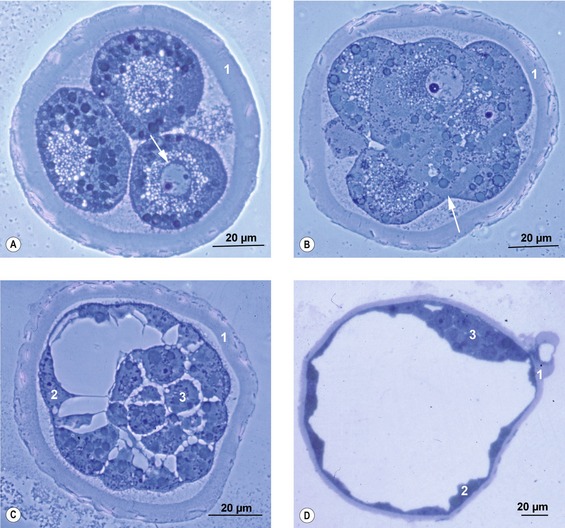
Individual cells of the morula all look identical to start with, their spherical shapes giving the morula its typical mulberry-like appearance. Later, however, the outer cells differentiate into an epithelium, attach firmly to each other, and give the embryo a smoother surface. This process is referred to as compaction (Fig. 6-3). The outer cells constitute the trophectoderm or trophoblast. In the present book, the term trophectoderm will be used before placentation and the term trophoblast when these cells become engaged in placental formation. The firm attachment between neighbouring cells of the trophectoderm results from specialized intercellular junctions including tight junctions and desmosomes. Hence, a typical epithelium with apical and basolateral cell compartments is formed. There are some species differences in the timing of compaction: in the pig it occurs very early in development, around the 8-cell stage, whereas in cattle it happens later, around the 16- to 32-cell stage.
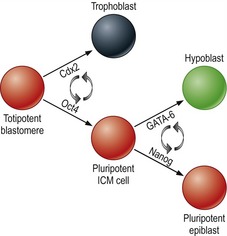
At the molecular level, differentiation of outer blastomeres, at least in the mouse, appears to rely on down-regulation of the transcription factor Oct4, followed by an up-regulation of other transcription factors such as Cdx2 and Eomesodermin (Fig. 6-4). The inner cells, meanwhile, retain expression of Oct4.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree