Chapter 32. Chronic Renal Failure
DESCRIPTION AND CLINICAL SIGNS
Chronic renal failure in dogs and cats is characterized by an irreversible and progressive loss of kidney function and the development of clinical signs that reflect the kidneys’ decreasing ability to perform normal regulatory and excretory functions. There are many potential causes for the initial kidney damage that leads to chronic disease. These causes include, but are not limited to, trauma, infection, immunological disease, neoplasms, renal ischemia (decreased blood flow to the kidneys), genetic anomalies, and exposure to toxins. Although renal disease can develop at any age, chronic renal disease is most frequently diagnosed in older pets. 1 In most cases the initial underlying cause of renal damage is no longer present when the pet develops chronic renal failure. This is due to the ability of the kidney to compensate for large proportions of functional tissue loss. However, over time these compensatory mechanisms may break down, leading to progressive loss of kidney function and signs of chronic disease.
Nephrons are the functional units of the kidneys. Each nephron consists of a glomerulus and a system of tubules within which reabsorption and excretion occur. The glomerulus is a tuft of capillaries where water, waste products, and electrolytes from the blood are filtered. The tubules originate at the base of the glomerulus and selectively reabsorb many of the blood components present in the filtrate. When the filtrate reaches the final portion of the tubule, it contains only those compounds that are going to be excreted as waste in the urine. The healthy kidney contains thousands of nephrons and has a substantial functional reserve.
Blood flow through the kidneys is very high, with approximately one fourth of cardiac output filtered through the kidneys each minute. The waste products of protein catabolism, such as urea, creatinine, uric acid, and ammonia are removed and excreted in the urine. In addition, electrolytes and trace minerals are filtered, reabsorbed, and selectively excreted. The kidneys are also important in the normal regulation of fluid balance, pH, and blood pressure and for the production of the hormone erythropoietin and the active form of vitamin D. All of these functions can be affected in pets with chronic renal disease.
The compensatory mechanisms of the healthy nephrons that remain after initial renal injury allow the kidneys to function normally even after the loss of a large proportion of tissue. A loss of at least 70% to 85% of functional capacity usually occurs before a pet begins to show clinical signs of renal failure. 1. and 2. One of the first signs that most pet owners notice is increased water consumption and increased urination. This effect is caused by a reduced capacity to concentrate urine, resulting in an increased volume of urine and increased frequency of urination. Some dogs may appear to regress in their house-training and begin to house-soil or involuntarily empty their bladder while sleeping. Polydipsia accompanies increased urination because the dog compensates with increased water consumption to maintain fluid balance. Polyuria and polydipsia are less commonly observed in cats because cats usually become uremic before they lose the ability to concentrate urine. 3 In addition, owners of indoor cats that use litter boxes are less likely to notice increased urination when it does occur.
The kidneys have an enormous reserve capacity; at least 70% to 85% of functional loss occurs before a pet begins to show clinical signs of renal failure. Common initial signs include increased water consumption and increased urination. Some dogs may appear to regress in their house-training and begin to house-soil or involuntarily empty their bladder while sleeping. Indoor cats begin to use the litter box more often, but this change may not be noticed by owners.
Many of the clinical signs seen in dogs and cats with advanced renal failure are associated with the degree of azotemia or uremia that is present. This is commonly referred to as uremic syndrome.4Azotemia refers to the accumulation of nitrogenous waste products in the blood, waste products composed primarily of urea nitrogen and/or creatinine. The term uremia technically means elevated concentrations of urea in the blood, but it commonly refers to the collection of clinical signs associated with renal failure. Although urea is singularly only a minor uremic toxin, serum urea levels are associated with the adverse clinical signs that reduce quality of life and contribute to morbidity in patients with chronic renal failure. 5 These signs include decreased appetite or anorexia, vomiting, depression, electrolyte and pH disturbances, mucosal ulcers, and weight loss. Some pets also develop chronic diarrhea and neurological signs. Aberrations in phosphorus and calcium metabolism lead to secondary renal hyperparathyroidism, which causes renal osteodystrophy (bone demineralization) and deposition of calcium phosphate in soft tissues. In many cases of chronic renal failure, the inability of the kidneys to produce erythropoietin and a reduced lifespan of red blood cells lead to the development of a normocytic, normochromic anemia (Box 32-1). 6
BOX 32-1
Polyuria
Increased frequency of urination
Polydipsia
Depression
Diarrhea
Vomiting
Anorexia
Renal osteodystrophy
Anemia
Neurological impairment
Diagnosis of chronic renal disease in dogs and cats is based on medical history, clinical signs, serum chemistry, and urinalysis. Pets with chronic renal failure develop elevated blood urea nitrogen (BUN) and plasma creatinine levels as a result of reduced glomerular function. Although BUN and creatinine measurements have been used for many years as the primary indicator of renal health in dogs and cats, they are relatively insensitive tests and do not accurately reflect the magnitude of renal functional loss. 7. and 8. This occurs because the relationship between glomerular filtration rate (GFR) and serum urea and creatinine concentrations is not linear, and up to 75% of renal function must be lost before BUN and creatinine values increase above the normal range. 7 After this point, values rise rapidly in response to small additional losses of renal function. Of the two measures, plasma creatinine is a more sensitive indicator of renal dysfunction and is not affected by dietary protein intake. In contrast, BUN is strongly affected by the consumption of a protein-containing meal. A fasting BUN of greater than 35 milligrams (mg)/deciliter (dl) may be an indication of some level of kidney dysfunction.
In 2006 the International Renal Interest Society (IRIS) established a set of diagnostic guidelines for dogs and cats with suspected chronic renal disease. 9 IRIS algorithms use fasting plasma creatinine level, presence of proteinuria, and systemic blood pressure to diagnose and stage kidney disease in dogs and cats. Separate algorithms are used for each species. Results allow practitioners to classify patients into stages and substages of renal disease, each of which is associated with a set of recommended treatment protocols. For example, plasma creatinine levels of between 2.1 and 5.0 mg/dl in dogs or between 2.9 and 5.0 in cats are indicative of moderate renal azotemia. Levels higher than 5.0, in either species, indicate severe renal azotemia that is typically associated with multiple extrarenal signs of disease. Tests for proteinuria and multiple blood pressure measurements are used to further classify animals into substages. A loss of concentrating ability and elevated serum phosphorus (greater than 5 mg/dl) also provides supportive evidence for a diagnosis of chronic renal failure. Laboratory test results may also show a normocytic, normochromic anemia; lymphopenia; hypercholesterolemia; or metabolic acidosis. Both lipase and amylase may be increased in the absence of pancreatitis due to decreased renal filtration.
With chronic renal disease, the gradual decline in GFR is responsible for the inability of the kidneys to filter and excrete waste products efficiently. However, early detection of kidney dysfunction is difficult in dogs and cats because clinical signs of uremia only develop after a large proportion of renal function has been lost. The most accurate and sensitive method for detecting early changes in renal function and for monitoring disease progression is to measure GFR. This test requires 24-hour urine collection by a veterinarian and measures the rates at which blood is filtered through the kidneys and waste products are removed and excreted. An estimate of GFR can also be obtained by measuring the renal clearance of exogenous creatine. 10. and 11. Unfortunately, these tests usually require expensive equipment and are time consuming and tedious to perform, so they are not available in most clinical settings. Efforts to produce a simpler and less expensive method for measuring GFR have reported success with using iohexol, an iodine-based radiographic contrast compound or gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA). 12.13. and 14. These tests may be especially important because early detection of a decreasing GFR can be the signal for nutritional interventions that can actually slow the progression of renal failure. Waiting for the appearance of azotemia or a loss of concentrating ability delays the use of these measures.
BUN and serum creatinine provide veterinary practitioners with a rapid screening test for assessing glomerular function in clinically affected patients. A fasting BUN of greater than 35 mg/dl is an indication of some level of kidney dysfunction. Plasma creatinine levels higher than 5.0 mg/dl indicate advanced failure or end-stage disease. IRIS guidelines also use blood pressure and presence of proteinuria to stage renal disease. The most accurate and sensitive method for detecting early changes in renal function is to measure GFR. Early detection of a decreasing GFR is important because it may allow early nutritional interventions that can slow the progression of renal failure.
PROGRESSIVE NATURE
The occurrence of chronic renal failure is preceded by some type of renal insult or injury that causes a loss of nephrons. Following this initial episode, the kidneys undergo structural and functional compensatory adaptations. Specifically, these changes include increased glomerular capillary hypertension, increased single nephron glomerular filtration rate (SNGFR), and renal hypertrophy (growth of remnant nephrons). 15. and 16. The increase in GFR in the surviving nephrons causes the kidneys’ total GFR to be higher than the level that would be predicted following the reduction in renal mass. These changes enable a damaged kidney to compensate and function for variable and extended periods of time at normal or near-normal capacity. During this compensatory phase, clinical signs of renal disease are not evident.
Depending on the extent of the damage to the kidneys and on other factors that can influence the progression of disease, renal function may eventually begin to decline. When this occurs, the progressive and irreversible loss of functioning nephrons causes a gradual reduction in total GFR and in the kidneys’ ability to excrete waste products from the body. The inability to excrete waste products and the compromised regulatory functioning of the kidneys lead to clinical signs of renal failure. Renal failure can progress to end-stage disease even after the initial cause of injury has been resolved and in the absence of active renal disease. It appears that the loss of a certain critical mass of nephrons can result in self-perpetuating, progressive renal disease. Studies in dogs have indicated that between ¾ and 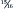 of renal mass must be destroyed before progression occurs. 17 However, great variability is seen among individuals. Some dogs and cats never develop progressive disease, even when an extremely high proportion of renal mass has been destroyed.
of renal mass must be destroyed before progression occurs. 17 However, great variability is seen among individuals. Some dogs and cats never develop progressive disease, even when an extremely high proportion of renal mass has been destroyed.
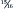 of renal mass must be destroyed before progression occurs. 17 However, great variability is seen among individuals. Some dogs and cats never develop progressive disease, even when an extremely high proportion of renal mass has been destroyed.
of renal mass must be destroyed before progression occurs. 17 However, great variability is seen among individuals. Some dogs and cats never develop progressive disease, even when an extremely high proportion of renal mass has been destroyed.Many factors may contribute to the progression of renal disease in dogs and cats. Evidence from early studies with rats suggested that the alterations that compensate for the initial loss of active tissue may eventually contribute to progressive deterioration of the remaining tissue. 18 These studies led to the hypothesis that hyperfiltration and hypertension of surviving nephrons ultimately would cause cellular injury, resulting in progressive glomerulosclerosis and a loss of nephron function. This hypothesis has been termed the hyperfiltration theory and appears to explain the progressive nature of renal disease in several strains of laboratory rat. 19. and 20. However, while glomerular hypertrophy and hypertension occur in dogs and cats with decreased renal function, the role of these changes in disease progression is unclear. 21. and 22. Studies with dogs indicate that the adaptive changes that occur following a loss of renal tissue do not lead to the progressive glomerulosclerosis that has been reported in rats. 23. and 24. In contrast, therapies that reduce glomerular hypertension have been shown to protect the kidneys from further damage in dogs with experimentally induced diabetic nephropathy. 25. and 26. These results suggest that a reduction in glomerular hypertension may be beneficial to dogs with chronic renal disease.
Role of Diet in Progression
Other factors that may contribute to spontaneous progression of renal disease in dogs and cats include systemic hypertension, hyperparathyroidism, intrarenal inflammation, hyperlipidemia, renal mineralization, and renal ammoniagenesis. Each of these factors is influenced to some degree by the animal’s diet and nutritional status. Chronically elevated serum phosphate levels can contribute to hyperparathyroidism and renal mineralization; dietary fatty acids affect serum lipid levels, intrarenal blood pressure, and inflammation; dietary sodium may influence the development of systemic hypertension; and metabolic acidosis affects ammonia production in the kidneys.
The goals of dietary management of chronic renal failure are to ameliorate clinical signs of uremia (see p. 417) and, if possible, slow or stop the progression of the disease. Historically, dietary protein was the major nutrient identified as important in slowing disease progression. However, studies have shown that protein is not an important factor in disease progression in dogs and cats, while other nutrients play a more significant role. Dietary components that can influence the rate of progression of chronic renal failure in dogs and cats include phosphorus, the type of fatty acids included in the diet, and nutritional factors that affect the body’s acid-base status.
PROTEIN
Feeding a high-protein diet results in increased renal blood flow and increased postprandial GFR in all species that have been studied, including the dog. 19. and 27. This effect is seen in both healthy animals and animals with compromised kidney function. A series of early studies reported that dietary protein restriction reduced these effects and slowed the progression of chronic renal disease in a susceptible strain of male rats with experimentally reduced renal mass. 19 Restricting dietary protein also slowed the development of progressive disease in healthy Fischer 344 rats that were genetically predisposed to develop chronic renal disease as they aged. 28. and 29.
These effects have not been observed in dogs. In contrast to rats, feeding elevated protein levels has not been shown to cause a progression of renal disease in dogs with either experimentally induced or naturally occurring renal disease. 23.24.30.31. and 32. In an early long-term study, diets containing either 19%, 27%, or 56% protein were fed to dogs with experimentally induced ¾ reduction in renal mass for a period of 4 years. 30 The dogs that were fed the high-protein diet (56% protein) had higher GFR and renal plasma flow rates than the dogs that were fed the low-protein diet (19% protein). However, significant morphological or functional deterioration in the remaining nephrons of the kidneys was not observed in any dogs. The investigators were unable to establish a cause and effect relationship between protein feeding and the progression of renal disease in the dogs that were studied. Feeding the high-protein (56%) and the low-protein (19%) diets was associated with slight proteinuria, but the 27%-protein diet did not cause this effect. In contrast to the rats that were studied, none of the dogs in this study developed elevated BUN levels or clinical signs of chronic renal disease in response to consuming moderate- or high-protein diets.
In another study, three groups of dogs with induced renal failure were fed three diets varying in protein, fat, carbohydrate, and mineral content. 32 Over a 40-week period, dogs that were fed the high-protein, high-phosphorus diet (44.4% protein, 2.05% phosphorus) showed the highest mortality rate. However, mortality was associated with uremia caused by the increased protein, rather than uremia caused by the development of progressive nephron destruction. There was no evidence of a decline in GFR (an indication of progressive renal disease) in the dogs fed the high-protein diet. These results indicate that the increased mortality was caused by the extrarenal, clinical effects of feeding high amounts of protein to dogs with renal failure and not to an enhanced progression of renal disease caused by the diet.
The previous studies do not support the hypothesis that protein affects the progression of chronic renal failure in dogs. Additional evidence comes from studies that fail to demonstrate that restricting dietary protein inhibits the initial development or progression of renal disease. Early studies with rats reported that feeding low-protein diets prolonged life and delayed the development of chronic renal disease. 19.33. and 34. However, follow-up studies found that benefits that had been attributed to low protein were actually a result of low energy intake throughout life. 29. and 35. Reduced energy consumption significantly slowed growth (which continues throughout life in rats) and retarded the progression of chronic renal disease. Unfortunately, the belief that feeding a low-protein diet prevents the development and the progression of renal disease had already been applied to several other species, including companion animals. This theory is without supportive scientific evidence in either dogs or cats.
In dogs, moderate restriction of dietary protein is not effective in modifying glomerular hypertrophy after a loss of kidney function. When dogs with a 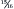 loss of kidney function were fed a moderately restricted diet containing 16% protein, the adaptive changes of hyperfiltration, capillary hypertension, and glomerular hypertrophy still occurred. 15 A second study of dogs with a ⅞ loss of functional kidney tissue reported that renal lesions were indistinguishable between dogs that were fed a diet containing 15% protein and those that were fed a diet containing 31% protein over a 14-month period. 36
loss of kidney function were fed a moderately restricted diet containing 16% protein, the adaptive changes of hyperfiltration, capillary hypertension, and glomerular hypertrophy still occurred. 15 A second study of dogs with a ⅞ loss of functional kidney tissue reported that renal lesions were indistinguishable between dogs that were fed a diet containing 15% protein and those that were fed a diet containing 31% protein over a 14-month period. 36
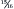 loss of kidney function were fed a moderately restricted diet containing 16% protein, the adaptive changes of hyperfiltration, capillary hypertension, and glomerular hypertrophy still occurred. 15 A second study of dogs with a ⅞ loss of functional kidney tissue reported that renal lesions were indistinguishable between dogs that were fed a diet containing 15% protein and those that were fed a diet containing 31% protein over a 14-month period. 36
loss of kidney function were fed a moderately restricted diet containing 16% protein, the adaptive changes of hyperfiltration, capillary hypertension, and glomerular hypertrophy still occurred. 15 A second study of dogs with a ⅞ loss of functional kidney tissue reported that renal lesions were indistinguishable between dogs that were fed a diet containing 15% protein and those that were fed a diet containing 31% protein over a 14-month period. 36Studies with cats have reported similar results. When cats with experimentally induced renal failure were fed either a high-protein diet (51.7%) or a low-protein diet (27.6%), cats consuming the low-protein diet showed fewer and less severe glomerular lesions in the remnant kidney. 37 However, the low-protein diet was less palatable, and cats consuming this diet had significantly lower caloric intakes than those consuming the high-protein diet, leading to weight loss and signs of protein deficiency. Because of this confounding effect, the authors of the study concluded that restriction of calories and protein led to less glomerular injury in cats with induced renal failure when compared with cats fed a diet replete in calories and protein.
A subsequent study was undertaken to elucidate the separate effects of protein and calorie intake on the progression of renal disease in cats. 38 A group of 28 adult female cats with experimentally induced renal failure was divided into four groups according to initial GFR. Each group received one of four diets for a 12-month period: low protein/low calorie, low protein/high calorie, high protein/low calorie, or high protein/high calorie. GFR did not decrease in any of the groups during the 12-month study period. Mild to moderate renal glomerular lesions were observed in all groups, but their development was not affected by either protein level or caloric intake. On the other hand, nonglomerular lesions in the kidneys were reported in cats fed the high-calorie diets but not in those fed the high-protein diets. The cat appears to be similar to the dog in that feeding a diet containing adequate protein does not exacerbate chronic renal disease.
Because of the early research studies with rats and because it has been hypothesized that elderly pets experience some loss of renal function as a normal process of aging, it became popular during the 1980s to advocate feeding low-protein diets to older animals, with the intent of slowing the rate of renal deterioration. However, no research has shown that there is an obligatory loss of kidney function with aging in either dogs or cats. 39.40. and 41. Elderly pets require adequate levels of high-quality protein to help to minimize losses of protein reserves and satisfy their maintenance needs (see Section 4, pp. 268-270). Long-term restriction of dietary protein in both dogs and cats is associated with several inherent dangers. Protein deficiency results in impaired immunological response and resistance to infection, reduced hemoglobin production and anemia, decreased plasma protein levels, and muscle wasting. 42. and 43. Restricting protein in older pets when it is not necessary can lead to further loss of protein reserves, malnutrition, and clinical signs associated with protein or amino acid deficiency. Finally, foods with severely reduced protein are low in palatability, which can lead to reduced intake that can further exacerbate protein deficiency. For example, when a diet containing 27.6% protein was fed to cats with ⅚ renal ablation, the cats consumed significantly less food when compared with cats fed 51.7% protein. 37 The cats fed the low-protein diet lost weight and developed hypoalbuminemia, a clinical sign of protein malnutrition. In contrast, cats fed the high-protein diet gained weight and did not develop protein deficiency. Consideration of the detrimental effects of protein restriction is particularly important in cats because this species does not readily adapt to reduced-protein diets (see Section 2, pp. 92-95).
Current evidence suggests that mechanisms that can alter the progression of renal disease in the rat do not have the same effect in the dog and cat. Dogs appear to be resistant to the glomerulosclerosis and loss of renal function associated with aging and adaptive changes in nephrons and protein-feeding in the rat. 39 Although high-protein feeding can exacerbate clinical signs by leading to azotemia in dogs with advanced renal failure, these effects occur because the loss of renal function leads to an accumulation in the blood of nitrogenous and nonnitrogenous end products of protein metabolism, not due to direct damage to the kidneys. 44 There is no evidence that dietary protein causes a progressive destruction of nephron functioning in the remaining normal tissue. It is hypothesized that the absence of systemic hypertension in dogs with renal disease and the fact that dogs do not normally develop the same type of renal disease as rats explain the differences between dogs and rats. In addition, unlike the rat, the dog does not continue to grow throughout its life and typically consumes only one to two meals per day, compared with the nibbling regimen of the rat. In dogs, hyperfiltration following the consumption of a meal that contains protein lasts for a short time, as opposed to continuously throughout a 24-hour period in the rat. The cat appears to be similar to the dog; routine restriction of protein in cats with chronic renal disease with the intent of slowing disease progression is not supported.
Dietary protein is not a contributor to either the initiation or progression of chronic renal disease in dogs and cats. Although high-protein feeding can exacerbate clinical signs by leading to azotemia in animals with advanced renal failure, these effects occur because the loss of renal function leads to an accumulation in the blood of nitrogenous and nonnitrogenous end products of protein metabolism, not due to increased damage to the kidneys.
PHOSPHORUS
A dietary factor that is involved in the progression of renal disease in companion animals is the level of phosphorus in the diet. As chronic renal failure progresses, GFR declines and leads to a decreased ability to excrete phosphorus. Declining kidney function also leads to an inability to produce calcitriol (active vitamin D) and to degrade parathyroid hormone (PTH). Collectively, these changes result in aberrations in phosphorus and calcium metabolism and can ultimately result in hyperphosphatemia, bone demineralization (osteodystrophy), and the deposition of calcium phosphate crystals in soft tissues. The deposition of calcium and phosphorus in renal tissue causes inflammation, scarring, and subsequent loss of nephrons. 45 Therefore the restriction of dietary phosphorus may help to control renal secondary hyperparathyroidism, resulting in decreased mineralization and less damage to the remaining functioning nephrons, ultimately slowing the progression of renal disease.
Studies with rats have shown that phosphorus restriction is effective in minimizing or preventing proteinuria and in slowing the structural and functional changes that occur in the remaining healthy nephrons. 46 Similar studies with dogs have found that the dietary restriction of phosphorus can slow the progression of clinical disease and prolong survival in azotemic dogs with induced chronic renal failure. 47 In addition, when diets containing 32% protein and varying levels of phosphorus were fed to dogs with induced renal failure, the dogs that were fed the low-phosphorus diets had significantly higher GFR values when compared with dogs fed the high-phosphorus diets. 48 However, over time, renal lesions developed that were not influenced by the level of phosphorus in the diet, indicating that other factors were involved in the progression of the disease. Beneficial effects of phosphorus restriction that are independent of the level of protein in the diet were found in a study comparing the effects of high and low dietary protein and phosphorus in dogs with a  reduction in renal mass. 49 Survival time and GFR stability were enhanced in dogs fed reduced phosphorus (0.4%) but were not affected by the level of protein fed. There is also evidence that normal phosphorus intake in cats with induced renal disease causes increased mineralization of renal tissue, and that dietary restriction can prevent these changes. 50 Current evidence suggests that high dietary phosphorus contributes to the progression of renal disease in dogs and cats and that phosphorus restriction can reduce damage to renal tissue and disease progression (see p. 420). However, because other factors are involved, progression of disease can still occur even with restricted dietary phosphorus.
reduction in renal mass. 49 Survival time and GFR stability were enhanced in dogs fed reduced phosphorus (0.4%) but were not affected by the level of protein fed. There is also evidence that normal phosphorus intake in cats with induced renal disease causes increased mineralization of renal tissue, and that dietary restriction can prevent these changes. 50 Current evidence suggests that high dietary phosphorus contributes to the progression of renal disease in dogs and cats and that phosphorus restriction can reduce damage to renal tissue and disease progression (see p. 420). However, because other factors are involved, progression of disease can still occur even with restricted dietary phosphorus.
 reduction in renal mass. 49 Survival time and GFR stability were enhanced in dogs fed reduced phosphorus (0.4%) but were not affected by the level of protein fed. There is also evidence that normal phosphorus intake in cats with induced renal disease causes increased mineralization of renal tissue, and that dietary restriction can prevent these changes. 50 Current evidence suggests that high dietary phosphorus contributes to the progression of renal disease in dogs and cats and that phosphorus restriction can reduce damage to renal tissue and disease progression (see p. 420). However, because other factors are involved, progression of disease can still occur even with restricted dietary phosphorus.
reduction in renal mass. 49 Survival time and GFR stability were enhanced in dogs fed reduced phosphorus (0.4%) but were not affected by the level of protein fed. There is also evidence that normal phosphorus intake in cats with induced renal disease causes increased mineralization of renal tissue, and that dietary restriction can prevent these changes. 50 Current evidence suggests that high dietary phosphorus contributes to the progression of renal disease in dogs and cats and that phosphorus restriction can reduce damage to renal tissue and disease progression (see p. 420). However, because other factors are involved, progression of disease can still occur even with restricted dietary phosphorus.A dietary factor that is involved in the progression of renal disease in companion animals is the level of phosphorus in the diet. Declining kidney function leads to an inability to excrete phosphorus, produce calcitriol, and degrade parathyroid hormone. Restriction of dietary phosphorus helps to control renal secondary hyperparathyroidism, resulting in decreased mineralization and less damage to the remaining functioning nephrons, ultimately slowing the progression of renal disease.
DIETARY LIPIDS
The amount and type of fat in a pet’s diet may affect the progression of renal disease. Hyperlipidemia has been identified as a causal factor in uremic renal failure in some species. 51. and 52. Like rats and humans, dogs and cats with renal dysfunction often exhibit elevated serum cholesterol and triglycerides. In addition, the degree of hyperlipidemia has been shown to be directly related to further losses of renal function in dogs with experimentally induced renal disease. When dogs with induced renal failure were fed a diet enriched in polyunsaturated fatty acids (PUFAs) (safflower oil or menhaden fish oil), they had lower blood lipid levels, compared with dogs fed a diet containing saturated fat. 53 These results indicate that replacing a proportion of saturated fat with polyunsaturated fat may be helpful in ameliorating the hyperlipidemia seen in pets with chronic renal disease.
A second factor affecting the progression of renal disease is the presence of increased vascular pressure in the kidneys, specifically within the glomerular capillaries. In rats, manipulations that increase glomerular pressure contribute to the progression of chronic renal failure, and factors that reduce glomerular hypertension are renoprotective. 19. and 54. Modification of the level of omega-3 fatty acids in the diet affects glomerular pressure and the progression of renal disease in rats. However, while some researchers report benefits of feeding omega-3 fatty acids, others found that feeding this class of fatty acid was associated with a worsening of disease in rats. 55.56. and 57. Similarly, while some human patients with immune-mediated forms of renal disease have benefited from supplementation with omega-3 fatty acids, others have shown no change in condition. 58. and 59.
Like rats and humans, dogs and cats with chronic renal disease develop glomerular hypertension. 16. and 21. A study of diabetic dogs demonstrated that therapies aimed at reducing glomerular hypertension significantly slowed the progression of kidney disease. 25. and 26. It is speculated that lowering glomerular pressure may also be of benefit to dogs and cats with other forms of chronic renal failure. 55 Dietary lipids influence intrarenal pressure through the effects of renal eicosanoid metabolism. Eicosanoids, produced from fatty acids, are one of several mediators of inflammation in many different tissues of the body (see pp. 387-390). During an inflammatory response, the release and metabolism of omega-6 fatty acids produces the 2-series prostaglandins, the 4-series leukotrienes, hydroxyeicosatetraenoic acid, and thromboxane A 2 (TXA 2). Two omega-6–derived eicosanoids, prostaglandin E 2 (PGE 2) and prostacyclin, are vasodilatory and proinflammatory and function in the kidney to increase renal blood flow and GFR. In contrast, TXA 2 causes vasoconstriction and has variable effects on GFR. Omega-3 fatty acid–derived eicosanoids are less potent inflammatory agents, and omega-3–derived thromboxanes have less vasoconstrictive and platelet-aggregating effects. Because omega-6 and omega-3 fatty acids compete for the same enzyme systems, increasing tissue concentrations of omega-3 fatty acids causes a diminution of the 2-series eicosanoids derived from the omega-6 fatty acid arachidonic acid, thus down-regulating intrarenal inflammatory responses.
A link between production of the 2-series of prostaglandins and thromboxanes and progressive renal disease has been proposed. 60 This theory is based on studies that suggest glomerular hypertension is affected by renal eicosanoids, and that supplementation with omega-3 fatty acids (marine fish oil) can reduce renal hypertension and may slow the progression of chronic renal disease. 61 One study measured the effects of three different types of fat on GFR in dogs with induced renal failure. 62 Dogs were fed a low-fat diet supplemented with either menhaden fish oil (a source of omega-3 fatty acids), safflower oil (a source of unsaturated omega-6 fatty acids) or beef tallow (a source of saturated omega-6 fatty acids) for a period of 20 months. Dogs fed the menhaden fish oil–supplemented diet had reduced proteinuria and lower serum creatinine, cholesterol, and triglyceride values when compared with dogs fed either safflower oil or beef tallow. While six out of seven dogs fed the diet enriched with omega-6 fatty acids showed progressive loss of renal function over the 20-week period, dogs fed the diet supplemented with omega-3 fatty acids did not exhibit signs of progression and actually had GFR test values indicating a slight increase in renal function at the end of the trial (Figure 32-1). These results suggested that omega-3 fatty acids may be renoprotective, while supplementation with omega-6 fatty acids may contribute to disease progression.
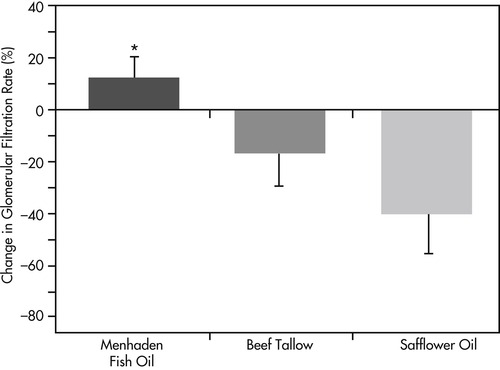 |
| Figure 32-1 (From New concepts in management of renal failure, Dayton, Ohio, 1998, The Iams Company.) |
A second study examined the effects of fatty acid supplementation in dogs with naturally occurring chronic renal failure. 63 Dogs were fed fatty acid supplements containing either safflower oil or menhaden fish oil for a 6-week period. Dogs fed the omega-3 supplement maintained GFR for the entire 6-week period and exhibited decreased levels of urinary PGE 2. The dogs supplemented with safflower oil had increased GFR and increased urinary PGE 2 concentrations. Although dogs fed safflower oil had higher GFR values, it is theorized that this was a short-lived effect caused by increased renal blood flow. The rise in PGE 2 is indicative of increased intrarenal pressure, which over time may contribute to the progression of disease.
OTHER CONTRIBUTING NUTRIENTS
A high level of dietary sodium has been identified as a potentially exacerbating factor in progression of chronic renal failure through enhancement of systemic hypertension. There is evidence in rats that sodium restriction can slow the progression of chronic renal disease. 64 For this reason, moderate sodium restriction has been recommended for dogs and cats with chronic renal failure. However, sodium restriction is not universally accepted because systemic hypertension is not a consistent finding in dogs and cats with renal failure. In addition, studies of risk factors and of the effects of reduced sodium intake in dogs and cats with compromised renal function have not shown a clear benefit of sodium restriction. 65.66. and 67. The unnecessary restriction of dietary sodium may exacerbate compromised renal concentrating ability due to reduced intrarenal sodium content, and may cause increased production of angiotensin in response to lowered systemic levels of sodium. Because the ideal dietary sodium intake for pets with chronic renal failure is not well defined and because of the risks of severe restriction, current recommendations are to provide a food that contains normal or moderately restricted amounts of sodium to pets with chronic renal disease (see p. 423). 68
Metabolic acidosis occurs commonly in pets with chronic renal failure and is caused by the reduced ability of the kidneys to excrete acids. For example, a retrospective study of cats with naturally occurring renal failure reported that approximately 80% of cats had metabolic acidosis at the time of diagnosis. 69 The development of acidosis in cats may be exacerbated by the acidifying nature of many commercial maintenance diets. Acidosis contributes to renal injury and uremia and leads to increased renal tubular generation of ammonia. These changes may all contribute to a progressive loss of renal function. In addition, there is an apparent association between metabolic acidosis and negative potassium balance in cats, which may further contribute to disease progression. 70
DIETARY MANAGEMENT
When chronic renal disease has been diagnosed in a dog or cat, medical management is directed toward normalizing blood pressure, preventing or reducing proteinuria, normalizing plasma phosphate (if dietary restriction is not sufficient), and preventing or correcting metabolic acidosis. 9 Dietary management is implemented with the goals of minimizing the clinical, biochemical, and physiological consequences of the loss of kidney function. Alterations in the kidneys’ ability to excrete waste products and to regulate metabolism of certain nutrients and hormones are the cause of the clinical signs that the animal experiences. Although dietary therapy does not cure chronic renal disease, it can minimize clinical signs and contribute to a pet’s health, well-being, and longevity. In addition, the modification of certain essential nutrients in the diet may slow the progression of disease. A major goal of dietary management is to minimize the accumulation of protein catabolites in the blood while still providing adequate protein for the pet’s maintenance needs. To this end, adequate calories from nonprotein sources must be provided to minimize the use of either body tissues or dietary protein for energy. Other nutrients of concern include dietary fat, fiber, phosphorus, sodium, potassium, and water-soluble vitamins (Box 32-2). In all cases, medical and dietary management should be customized to address the needs of the individual patient and modified as a patient’s stage of disease changes. 9
BOX 32-2



 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Maintain nitrogen balance by providing optimal protein nutrition.
Provide adequate nonprotein calories.
Minimize azotemia and associated clinical signs.
Normalize serum phosphorus.
Normalize blood pH.
Normalize electrolyte balance and good hydration.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


