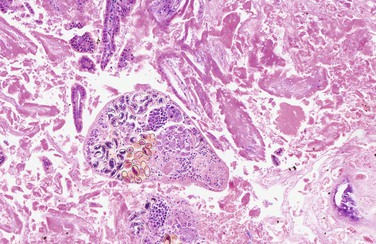
Elizabeth L. Buckles Bats have always fascinated people. Unfortunately, much of the connotations around them have been negative. Their odd appearances, nocturnal life styles, and the fact they harbor a variety of zoonotic diseases, particularly rabies, have lead them to be feared rather than respected. However, beneath the surface of the legend, they are members of a diverse group of animals, many of which are essential for the maintenance of ecosystems. In fact, because of disease and habitat destruction, humans are a greater threat to bats than they are to humans.68 Recent events such as the emergence of white nose syndrome (WNS) and the continued recognition of metabolic diseases in captive bats have shown that much more research is needed into the medical and environmental needs of these animals. Improving veterinary care, conservation efforts, and captive husbandry relies on a deeper understanding of bat biology and how these unique animals fit into larger ecosystems. What is known about bats is that they belong to the order Chiroptera, literally meaning “hand wing.” Some mammals such as lemurs and squirrels may parachute, but only bats have powered flight. It is this unique anatomic feature and the ability to fly that distinguish bats from all other mammals. Their wings are actually modifications of the standard mammalian hand consisting of elongate, slender phalanges spanned by a thin, tough wing membrane that serves as a flexible airfoil.38 This anatomy lends a high degree of adaptability and maneuverability to the flight of bats and allows fine adjustments to navigation. Ancestors of modern bats first appeared, as evidenced by fossil records, approximately 53 million years ago. As is the case today, ancestral bats were distributed worldwide and appear to have been capable of flight. Today, at least 1240 known species of bats exist, accounting for 20% of all known mammals. They are found on all continents except Antarctica and inhabit a variety of environmental and dietary niches.38 Traditional subdivisions of Chiroptera are the two suborders Megachiroptera and Microchiroptera.38 Current theory suggests that both groups evolved from a common flighted ancestor, and this is generally supported by molecular evidence. Fossil evidence shows that these ancestral bats displayed many modern characteristics, including the ability to echolocate and specializations for fruit and nectar feeding.38 Despite the terminology, it is ecology and anatomy, not size, that distinguishes the groups. Megachiroptera include “fruit bats,” which eat fruit, nectar, and pollen. They are efficient climbers, as they have a claw on each wing, and have excellent eyesight. Microchiroptera species may feed on a variety of mammals, reptiles, fish, fruit nectar, and, in some cases, blood. Their eyesight is poor, and they have relatively small eyes. Hence they rely on echolocation for navigation and hunting.38 Bats are found in a variety of habitats, ranging from arid to tropical. Some have limited home ranges, and some range widely. Most species of bat are found in tropical or subtropical regions, where they may remain year round. Those inhabiting less temperate areas must adapt to survive inhospitable climates. Some species migrate, whereas others hibernate. Both survival strategies require specializations. Migratory bats have narrow, more pointed wings that provide more efficient wing strokes for sustained flight. Both hibernating and migratory bats have developed life histories, which allow them to increase fat store prior to hibernation, and thus rely on abundant steady food supplies during certain times of the year.38 Hibernating bats undergo profound physiologic alterations during hibernation, which result in decreased metabolism, body temperature, and modulation of the immune system. Having an abundant food supply available after hibernation is essential for the survival of these bats.38 The diets and sizes of modern bats are as diverse as their habitats. At one end of the size spectrum is the Kitti’s hog-nosed bat (Craseonycteris thonglongyai). This bat may be the world’s smallest mammal, with a length of 29 to 33 millimeters (mm) and weighing a mere 2 to 2.6 grams (g).52,67 Also known as the bumblebee bat, it is native to the limestone caves in a small riparian area of Thailand and Burma. At the other end of the spectrum is the giant golden-crowned flying fox (Acerodon jubatus), which weighs 1.1 to 1.2 kilograms (kg). Unlike the insectivorous bumblebee bat, this bat is widely prevalent through the Filipino rainforests, where it feeds primarily on figs. Both the flying fox and the bumblebee bat rely heavily on their unique environments, and both are threatened by deforestation and other anthropogenic alterations to their habitats.52,67 Approximately 70% of known Chiroptera are insectivorous, and most of the other species are fruit eaters. Insectivorous bats may either hunt their prey on the wing or glean insects from plants.38,52,67 This distinction becomes important during attempts to get an insectivorous species to adapt to a captive diet. More species with specialized diets include picivorous bats such as the fish-eating myotis (Myotis vivesi) and the greater bulldog (Noctilio leporinus) bat. The highly specialized vampire bats, including the hairy-legged vampire bat (Diphylla ecaudata), white-winged vampire bat (Diamus youngi), and the common vampire bat (Desmodus rotundus), are hematophagous and are the only known mammals that are obligate parasites.38 Fruit-eating bats may limit their diets to a small number of fruits, whereas others are more generalized feeders.38 When designing plans for conservation or for sustaining bats in captivity, it is essential that the normal wild feeding habits of each species be understood so as to provide the proper diet and feeding opportunities. Regardless of size, bats have the potential to cause injury to handlers. All species of bats have sharp teeth, and many have sharp claws. The degree of physical damage that can be inflicted does depend on the size of the animal, so the extent of personal protective gear depends on the species. At the very least, sturdy, bite-resistant gloves should be worn while handling these animals. Moreover, bats have the potential to spread a variety of zoonotic diseases. The classic bat-associated disease is rabies, but other diseases can also be transmitted. Lysaviruses, filoviruses, and various fungi and bacteria can be potentially spread to humans from bats. Individuals working with bats should know the risks posed by the species in question and should be vaccinated for rabies virus and have their antibody titers checked regularly. Besides wearing gloves, other precautions include proper training to prevent bites and scratches and wearing personal protective equipment to prevent exposure to aerosols, urine, and feces. These might include goggles, face shields, or both, moisture resistant clothing, and boots that can be washed and disinfected. Individuals performing medical procedures or postmortem examinations on bats should take precautions to avoid needlesticks and ensure that all medical waste, sharps, and equipment are properly disposed of. Care should also be taken to avoid injuring the bat during handling. Respiratory function of small bats can be compromised by excessive pressure on the chest or neck. The wings of all bats should be cared for during handling. This includes ensuring that the wings are properly immobilized to prevent fractures and that the wing membranes are protected, as these can be torn and may expose the animal to infection and possibly prevent proper mobility. Proper biosecurity should also be observed when handling both free-ranging and captive bats. In captivity, care must be taken to avoid exposing bats to potentially infectious material from other captive species or having them come in contact with free-ranging bats or other wildlife. In the wild, equipment should be disinfected between visits to different habitats and even between individual bats if infectious diseases are a concern. Biosecurity during fieldwork has become an important part of the management strategy during outbreaks of WNS in North America and is one way officials are trying to prevent the spread of the associated fungus. Megachiroptera are a familiar part of many zoologic collections as popular inhabitants of nocturnal displays. Species such as Egyptian fruit bats are often housed in groups, and their unique appearance and active behaviors make them attractive to the public. Even the larger, more solitary flying foxes may be found in some of the more complete rainforest displays. Despite the fact that these animals are frequently housed in captivity, much needs to be learned about proper husbandry of these animals. What is known is that the husbandry and dietary needs of Megachiroptera are as diverse as the species involved. Thus, a full description of the needs of each species is beyond the scope of this chapter. Moreover, the regulations for housing these animals are also diverse and dependent on government regulations and the type of facility in which the bats are kept. Prior to housing any animals, local regulations governing the maintenance of animals in the facility should be consulted. In general, as with many nondomestic animals, captive management should be guided by knowledge of the natural history of the species in the wild as well as modifications to account for the captive environment. Factors to consider include the optimal stocking density for a given enclosure, natural photoperiod of the animal, diet and feeding schedule, natural temperature ranges, size of the animal, and cleaning needs of the exhibit. Providing adequate room for the animals to fly is a major consideration. Not only do bats need facilities that allow flight, they must also be housed in facilities from where they cannot easily escape when personnel need access to feed or clean the exhibit. Safety features such as double doors are highly recommended. Although the ability to provide enough room for sustained flight might be limited by the size of the animal, they should be able to spread their wings and move about the enclosure. Care should be taken to ensure that the animals have enough room to avoid damaging their wing membranes or bones. Species that are known climbers should be provided with branches and other materials on which to climb or suspend themselves. The animals often rest in an inverted position, so they should have enough room to do so without their heads, ears, or edges of the wings contacting substrates, as this might cause trauma that could result in open wounds susceptible to infection.27,28 With regard to temperature and humidity, no hard and fast rules exist for the temperature at which an enclosure should be maintained. Many bats may withstand a wide range of temperatures and humidities, but efforts should be made to ensure a constant range, as many of these species are tropical and may not be able to tolerate low temperatures or low humidity for sustained periods. Temperatures between 18° C and 27° C are reported as being adequate, but this may vary with species.27,28 Dietary requirements are also diverse. Flying foxes prefer sweet, soft fruits and may be maintained on such foods supplemented with vitamins, bone meal, and milk replacer. However, the actual dietary requirements of many captive species are unclear. The American Zoo and Aquarium Association Chiropteran Taxon Advisory Group provides guidelines for the nutrient and mineral content of fruit bat diet, and many captive colonies of fruit bats thrive on diets based on these guidelines.19 Nonetheless, more data on nutrient intake and utilization are required to further refine the nutritional recommendations for captive fruit bats and establish the suitability of these recommendations for different species. Several studies have examined captive diets and have focused on determining if what the bats consume provides the recommended amount of nutrients. Importantly, predicted dietary values and actual dietary values may differ. Specifically, vitamins A and E and cascium (Ca) concentrations have been found to be lower in the actual diet than would be predicted on the basis of the ingredients. Similarly, trace mineral concentrations of phosphorus (P), magnesium (Mg), and zinc (Zn) tend to be higher in left-over food than in the original diet and tend to be high in feces. This discrepancy suggests some dietary selection by the animals or that the animals are obtaining these elements from alternative sources such as from the galvanized materials in enclosures.23 Maintaining an overall healthy balance of the levels of vitamins in the diet is important, as complex interaction between compounds may affect the absorption and excretion of other nutrients. Deficiencies or excesses have been associated with the development of disease. Vitamin E deficiency has been associated with cardiomyopathy and excess flouride with a syndrome of hyperostosis.23,25 As with many frugivores, excess iron in the diet of fruit-eating bats may result in hepatic disease. When tissues levels of iron become high enough, hepatocytes are damaged, leading to hepatic necrosis and scarring of the liver in more chronic situations.23 This propensity to develop both hepatic hemosiderosis and hemochromatosis likely relates to the relative paucity of available iron in the diets of free-ranging animals. Frugivorous bats may particularly absorb iron efficiently, which leads to excessive absorption when iron levels are high. The regulation of iron levels is complex and is mediated not only by dietary levels but by interactions with other nutrients. High concentrations of vitamin C enhance the absorption of iron and may potentiate free radical damage to tissues.25 Furthermore, other dietary constituents found in the wild and not in captivity may serve to mediate iron availability. Tannins, calcium phosphate, egg yolk, and bran may inhibit iron absorption. Ferrous iron itself is transported across enterocyte membranes to the cytoplasm by divalent metal transporter 1 (DMT1). DMT1 may also transport other metal ions such as cobalt, lead, zinc, cadmium, and copper. The presence of these ions in some cases may upregulate DMT1 and thus secondarily lead to increased iron transport. Alternatively, the presence of these metals may competitively inhibit ferrous ion transport.23,25,40 Monitoring and treatment of iron storage disease is an important aspect of captive management of frugivorous bats. Although histopathology is the gold standard for the diagnosis of hemochromatosis, less invasive methods such as use of blood parameters may also be used to monitor the development of iron storage disease. Surprisingly, despite the hepatic damage caused by iron accumulation, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are not useful in detecting iron-induced liver damage. Farina (2005) studied the correlation of various blood parameters to histologic grade of iron deposition and damage in two species of fruit bat, the Egyptian fruit bat (Rousettus aegyptiacus), and the island flying fox (Pteropus hypomelanus).25 Serum iron, transferrin saturation, and plasma ferritin showed positive correlation with morphologic hepatic iron concentration.25 If the product of the serum iron level and the transferrin saturation exceeded 51, the bats had a high probability of iron overload. Products greater than 90 indicated a high probability of hemochromatosis. It has yet to be fully determined if these techniques may be used to monitor efficacy of treatment for iron overload.25 Management of iron overload begins with feeding low-iron diets and managing the levels of other vitamins and factors such as vitamin C that may enhance iron absorption. Unfortunately, maintaining low levels of vitamin C in a diet based on fresh fruit is difficult. Treatment for iron overload may also include addition of dietary chelators, phlebotomy, and in some cases an injectable chelator such as deferoxamine mesylate. Addition of even a small amount of tannic acid to the diet has been shown to reduce the absorption of iron up to 40% in captive straw-colored fruit bats (Eidolon helvum).40 However, as noted by Farina, tannins may be bitter and unpalatable and may not be accepted by all individuals.25 Like Megachiroptera, Microchiroptera species have widely variable habitats, natural histories, and nutritional needs. Compared with fruit bats, these animals are more rarely kept in captivity. Thus, knowledge of proper environmental and dietary management is scant. Depending on the natural habitat of the animal, needs for temperature, photoperiod, and humidity may vary widely.38 Many species may actively alter their body temperature and lower their metabolic rate, even to their own detriment, if ambient conditions are suboptimal.2,11 Thus, when housing these animals, it is advisable to provide them with a range of conditions to allow them to choose their own optimal microenvironment.27,28 As with Megachiroptera, Microchiroptera species rest by hanging by their feet. Thus, areas rough enough for them to grasp must be provided, but care must be taken that the caging material is not so rough that it causes injury to the animals. In some research settings, bats are provided with strips of cloth, and cloth bags are hung along the edges of the habitat to provide rest areas. Whatever is provided, it must be possible to clean it adequately (Buckles, personal observation). In some situations, inducing hibernation or torpor may be required. This may be a difficult endeavor. Some facilities have successfully housed hibernating bats in refrigerated enclosures. It is important to maintain proper humidity in these enclosures, as cold dry air may cause the bats to rapidly dehydrate. Improper humidity may also allow the bats’ wings to be infected by Pseudomonas and other bacterial species, causing death from sepsis or toxemia (Buckles, personal observation). Before attempting to have these animals hibernate in captivity, it is best to contact someone with previous experience for advice on the particular situation to maximize the survivability of the bats. Diet also presents a challenge in these species. Many of the Microchiroptera species are insectivorous, and in the wild, they catch food while in flight. Other insectivorous bats may pick food off a substrate. Adapting any insectivorous bat to a captive diet may require individual attention to each animal to ensure proper food intake.27,28 Some failures to adapt could be related to the fact that the movements of mealworms do not stimulate the natural prey recognition response. In one study, bats were more prone to accept food if artificial wings, mimicking the flapping frequency of the natural prey items were added to mealworms.17 Both obesity and starvation have been reported in captive insectivorous bats. In most cases, animals are fed diets consisting of artificially raised mealworm larvae. Since insectivorous bats will not accept supplementation with commercially available, nutritionally balanced diets, they are limited to this single source of food. The diets of free-ranging bats are composed of a much wider array of insects than is available in captivity and thus provide a more balanced and complete food source.17 The captive diets may be low in calcium and high in phosphorous. Nutrition may be improved if the insects being raised as a food source are placed on a mineral premix at least 24 hours before feeding them to the bats. In one dietary study, bone densities of captive mustached bats (Pteronotus parnelli rubiginosis), an insectivorous species from South America, maintained on unsupplemented insects were examined. The skulls of the captive animals were soft on palpation, and bone density was significantly lower than in free-ranging individuals.17 Once the mealworms were supplemented with calcium, the bone density of the animals increased, and no statistical difference between the bone density of captive animals fed calcium-supplemented mealworms and their free-ranging counterparts was observed.17 Even when on a calcium-supplemented diet, the captive animals tended to have less body mass compared with free-ranging bats. However, within the study group, some animals had significantly higher body weights than did other individuals. It is possible that some individuals adapt better to the captive lifestyle and monopolize the food supply. This in itself may present a problem, since mealworms are innately higher in fat than most insects, placing well-adapted animals at risk for obesity.17 Maintaining proper fat metabolism is particularly important in hibernating bats, as the fat depots laid down prior to entering hibernacula are their sole source of energy during this period. Alterations in fat metabolism during hibernation may lead to bats having insufficient energy to survive.10 Alternatively, altered fat mobilization caused by hibernation may also cause abnormal metabolism and deposition of lipid within hepatocytes, renal tubular epithelial cells, and myocardial cells. Such systemic lipidosis has been documented in greater horseshoe bats (Rhinolophus ferrumequinum) dying after transport during hibernation.31 Additionally, the female’s reproductive cycles are suppressed during hibernation by interactions of circulating leptin, insulin-like receptor, and insulin levels. This affects the amount of stored body fat, and changes in the normal lipid or hormonal environment may have adverse effects on the reproductive capability of bats after emergence from hibernation.61 Little is known about pathologic conditions in either Megachiroptera or Microchiroptera. Reports of disease are sporadic, and a great deal of the literature comprises surveys of wild animals that focus on pathogens of significance to human health rather than on disease states of the bats themselves. Sporadic, often anecdotal, reports of unexplained die-offs in fruit bats have been published. These include mortality events in Pacific flying foxes (Pteropus mariannus) in Micronesia, a mass mortality of insular flying foxes (P. tonganus) in Fiji, and die-offs of unspecified species of fruit bats on the Admiralty and Solomon Islands. No investigations were conducted as to the cause of these events, but in some cases, these die-offs coincided with outbreaks of infectious diseases such as measles in the local human population. In other cases, introduction of disease by domestic animals was suspected.38 Until the emergence of WNS in North America, reports of mass mortalities in Microchiroptera were rare and often attributed to rabies. In the mid-1980s, a thousand dead bats of various species, including Myotis spp. and Lassarius spp. were found dead in a Canadian lake along with dozens of dead mallards. The animals were in generally good health, and after laboratory tests confirmed the presence of toxic alkaloids, the deaths were attributed to blue-green algae toxicosis, as the algae was found covering the carcasses and in the water.55 Other causes of significant mortality have been related to barotrauma around wind farms because of bats being unable to navigate successfully past windmills.51 A particularly complete evaluation of causes of mortality of German bats has been published.48 Various bacterial infections, traumatic injuries, and parasitic infections, as well as physiologic diseases such as hypertension, were documented in this study. Additional studies correlated disease development with the physiology and ecology of the various bat species.48 Reports of fatal parasitic diseases are rare. However, anyone who has handled free-ranging bats knows that they often are infested with a variety of ectoparasites. In North America, ectoparasites include Myodopsylla insignnis, Spinturnix americanus, Cimex adjunctus, Macronyssu scrosbyi, and Adndrolaelab scasalis. The number of parasites on a bat may vary with roost size, energy status, and grooming efficacy.20 Ectoparasites have been reported on captive fruit bats, particularly those that have been recently captured. Demodex sp. have been documented in captive Egyptian fruit bats (R. aegyptiacus) as an incidental finding. A single wild-caught Egyptian fruit bat (Rousettus aegyptiacus leachi) was reported to be infected with a single Eucampsipoda africana, a nycteribiid ectoparasite.53 Although some ectoparasites do feed on blood, bats appear to be unaffected, and most infestations are self-limiting. This self-limiting nature is likely the immune response. Experimental studies of Serotine bats (Eptesicus serotinus) demonstrate that the bats develop a significant inflammatory response after the attachment of bat tick (Argas vespertilionis). The initial response consists of neutrophils followed by eosinophils and basophils and centers around the tick’s mouth parts. Adenosine triphosphatase (ATPase)–positive cells, presumed to be Langerhans cells, are frequently present in the lesions, and epithelial cell proliferation occurs into the tick mouthparts. It is presumed that this cellular and enzymatic environment is not suitable for the ticks and results in resistance to infection.21 A few surveys of wild populations have documented various blood parasites and flagellates. Schizotrypanum has been found in Kuhl’s pipistrelles (Pipistrellus kuhli). A trypanosome in the subgenus Megatrypanum and a parasite consistent with Herpetosoma has been found in naked-rumped tomb bats (Taphozous nudiventris).42 In one study, 16 bats incidentally caught in mist nets meant for birds in Zambia were examined, and 37.5% of these Gambian epaulated fruit bats (Epomophorus gambianus) were infected with Hepatocystis epomophori, a hematozoan parasite.53 Endoparasites are found incidentally in Microchiroptera. Digenean flukes, ascarids, cestodes, and coccidia are common intestinal inhabitants (Figure 35-1).24,41 Renal coccidia are sometimes encountered incidentally on histology.
Chiroptera (Bats)
Overview and Natural History
Handling, Captive Management, and Diet
Handling of Bats
Management of Megachiroptera
Management of Microchiroptera
Diseases of Chiroptera
Parasitic Infections
Chiroptera (Bats)
Chapter 35